Metabolic gene alterations impact the clinical aggressiveness and drug responses of 32 human cancers
- PMID: 31754644
- PMCID: PMC6856368
- DOI: 10.1038/s42003-019-0666-1
Metabolic gene alterations impact the clinical aggressiveness and drug responses of 32 human cancers
Abstract
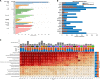
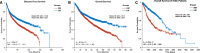
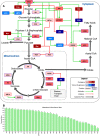
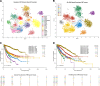
Malignant cells reconfigure their metabolism to support oncogenic processes such as accelerated growth and proliferation. The mechanisms by which this occurs likely involve alterations to genes that encode metabolic enzymes. Here, using genomics data for 10,528 tumours of 32 different cancer types, we characterise the alterations of genes involved in various metabolic pathways. We find that mutations and copy number variations of metabolic genes are pervasive across all human cancers. Based on the frequencies of metabolic gene alterations, we further find that there are two distinct cancer supertypes that tend to be associated with different clinical outcomes. By utilising the known dose-response profiles of 825 cancer cell lines, we infer that cancers belonging to these supertypes are likely to respond differently to various anticancer drugs. Collectively our analyses define the foundational metabolic features of different cancer supertypes and subtypes upon which discriminatory strategies for treating particular tumours could be constructed.
Keywords: Biochemical reaction networks; Cancer genomics; Data integration; Tumour heterogeneity.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures



References
-
- Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell144, 646–674 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

