Overexpression of Sirtuin 1 protein in neurons prevents and reverses experimental diabetic neuropathy
- PMID: 31754701
- PMCID: PMC6885680
- DOI: 10.1093/brain/awz324
Overexpression of Sirtuin 1 protein in neurons prevents and reverses experimental diabetic neuropathy
Abstract
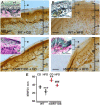
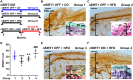
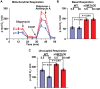
In diabetic neuropathy, there is activation of axonal and sensory neuronal degeneration pathways leading to distal axonopathy. The nicotinamide-adenine dinucleotide (NAD+)-dependent deacetylase enzyme, Sirtuin 1 (SIRT1), can prevent activation of these pathways and promote axonal regeneration. In this study, we tested whether increased expression of SIRT1 protein in sensory neurons prevents and reverses experimental diabetic neuropathy induced by a high fat diet (HFD). We generated a transgenic mouse that is inducible and overexpresses SIRT1 protein in neurons (nSIRT1OE Tg). Higher levels of SIRT1 protein were localized to cortical and hippocampal neuronal nuclei in the brain and in nuclei and cytoplasm of small to medium sized neurons in dorsal root ganglia. Wild-type and nSIRT1OE Tg mice were fed with either control diet (6.2% fat) or a HFD (36% fat) for 2 months. HFD-fed wild-type mice developed neuropathy as determined by abnormal motor and sensory nerve conduction velocity, mechanical allodynia, and loss of intraepidermal nerve fibres. In contrast, nSIRT1OE prevented a HFD-induced neuropathy despite the animals remaining hyperglycaemic. To test if nSIRT1OE would reverse HFD-induced neuropathy, nSIRT1OE was activated after mice developed peripheral neuropathy on a HFD. Two months after nSIRT1OE, we observed reversal of neuropathy and an increase in intraepidermal nerve fibre. Cultured adult dorsal root ganglion neurons from nSIRT1OE mice, maintained at high (30 mM) total glucose, showed higher basal and maximal respiratory capacity when compared to adult dorsal root ganglion neurons from wild-type mice. In dorsal root ganglion protein extracts from nSIRT1OE mice, the NAD+-consuming enzyme PARP1 was deactivated and the major deacetylated protein was identified to be an E3 protein ligase, NEDD4-1, a protein required for axonal growth, regeneration and proteostasis in neurodegenerative diseases. Our results indicate that nSIRT1OE prevents and reverses neuropathy. Increased mitochondrial respiratory capacity and NEDD4 activation was associated with increased axonal growth driven by neuronal overexpression of SIRT1. Therapies that regulate NAD+ and thereby target sirtuins may be beneficial in human diabetic sensory polyneuropathy.
Keywords: NAD+; NEDD4; diabetic neuropathy; mitochondria; sirtuins.
Published by Oxford University Press on behalf of the Guarantors of Brain 2019. This work is written by US Government employees and is in the public domain in the US.
Figures



References
-
- Aquilano K, Baldelli S, Pagliei B, Ciriolo MR. Extranuclear localization of SIRT1 and PGC-1α: an insight into possible roles in diseases associated with mitochondrial dysfunction. Curr Mol Med 2013; 13: 140–54. - PubMed
-
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 2004; 305: 1010–3. - PubMed
-
- Barbosa MT, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, et al.The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J 2007; 21: 3629–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

