The potassium channel Kcne3 is a VEGFA-inducible gene selectively expressed by vascular endothelial tip cells
- PMID: 31754927
- PMCID: PMC7160073
- DOI: 10.1007/s10456-019-09696-8
The potassium channel Kcne3 is a VEGFA-inducible gene selectively expressed by vascular endothelial tip cells
Abstract
Angiogenesis is largely driven by motile endothelial tip-cells capable of invading avascular tissue domains and enabling new vessel formation. Highly responsive to Vascular Endothelial Growth-Factor-A (VEGFA), endothelial tip-cells also suppress angiogenic sprouting in adjacent stalk cells, and thus have been a primary therapeutic focus in addressing neovascular pathologies. Surprisingly, however, there remains a paucity of specific endothelial tip-cell markers. Here, we employ transcriptional profiling and a lacZ reporter allele to identify Kcne3 as an early and selective endothelial tip-cell marker in multiple angiogenic contexts. In development, Kcne3 expression initiates during early phases of angiogenesis (E9) and remains specific to endothelial tip-cells, often adjacent to regions expressing VEGFA. Consistently, Kcne3 activation is highly responsive to exogenous VEGFA but maintains tip-cell specificity throughout normal retinal angiogenesis. We also demonstrate endothelial tip-cell selectivity of Kcne3 in several injury and tumor models. Together, our data show that Kcne3 is a unique marker of sprouting angiogenic tip-cells and offers new opportunities for investigating and targeting this cell type.
Keywords: Endothelial tip-cell; Kcne3; Retinal angiogenesis; VEGFA.
Figures
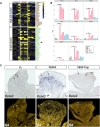
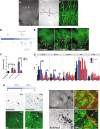
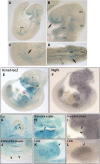
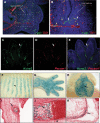

Similar articles
-
Notch pathway targets proangiogenic regulator Sox17 to restrict angiogenesis.Circ Res. 2014 Jul 7;115(2):215-26. doi: 10.1161/CIRCRESAHA.115.303142. Epub 2014 Apr 22. Circ Res. 2014. PMID: 24755984
-
MEF2 transcription factors are key regulators of sprouting angiogenesis.Genes Dev. 2016 Oct 15;30(20):2297-2309. doi: 10.1101/gad.290619.116. Genes Dev. 2016. PMID: 27898394 Free PMC article.
-
Endothelial-specific YY1 governs sprouting angiogenesis through directly interacting with RBPJ.Proc Natl Acad Sci U S A. 2020 Mar 3;117(9):4792-4801. doi: 10.1073/pnas.1916198117. Epub 2020 Feb 19. Proc Natl Acad Sci U S A. 2020. PMID: 32075915 Free PMC article.
-
Molecular control of endothelial cell behaviour during blood vessel morphogenesis.Nat Rev Mol Cell Biol. 2011 Aug 23;12(9):551-64. doi: 10.1038/nrm3176. Nat Rev Mol Cell Biol. 2011. PMID: 21860391 Free PMC article. Review.
-
Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review.
Cited by
-
Gene expression of intracortical bone demonstrates loading-induced increases in Wnt1 and Ngf and inhibition of bone remodeling processes.Bone. 2021 Sep;150:116019. doi: 10.1016/j.bone.2021.116019. Epub 2021 May 21. Bone. 2021. PMID: 34023542 Free PMC article.
-
Mst2 Overexpression Inhibits Thyroid Carcinoma Growth and Metastasis by Disrupting Mitochondrial Fitness and Endoplasmic Reticulum Homeostasis.J Oncol. 2021 Sep 6;2021:1262291. doi: 10.1155/2021/1262291. eCollection 2021. J Oncol. 2021. Retraction in: J Oncol. 2023 Oct 11;2023:9894235. doi: 10.1155/2023/9894235. PMID: 34557228 Free PMC article. Retracted.
-
Targeting FSCN1 with an oral small-molecule inhibitor for treating ocular neovascularization.J Transl Med. 2023 Aug 18;21(1):555. doi: 10.1186/s12967-023-04225-0. J Transl Med. 2023. PMID: 37596693 Free PMC article.
-
Succinate-induced macrophage polarization and RBP4 secretion promote vascular sprouting in ocular neovascularization.J Neuroinflammation. 2023 Dec 21;20(1):308. doi: 10.1186/s12974-023-02998-1. J Neuroinflammation. 2023. PMID: 38129891 Free PMC article.
-
Endothelial sprouting, proliferation, or senescence: tipping the balance from physiology to pathology.Cell Mol Life Sci. 2021 Feb;78(4):1329-1354. doi: 10.1007/s00018-020-03664-y. Epub 2020 Oct 19. Cell Mol Life Sci. 2021. PMID: 33078209 Free PMC article. Review.
References
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. - PubMed
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. - PubMed
-
- Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21(20):2511–2524. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

