Adjusting Overall Survival Estimates for Treatment Switching in Metastatic, Castration-Sensitive Prostate Cancer: Results from the LATITUDE Study
- PMID: 31754962
- PMCID: PMC6875513
- DOI: 10.1007/s11523-019-00685-x
Adjusting Overall Survival Estimates for Treatment Switching in Metastatic, Castration-Sensitive Prostate Cancer: Results from the LATITUDE Study
Abstract
Background: LATITUDE was the first phase 3 trial examining the survival benefit of adding abiraterone acetate (AA) + prednisone (P) to androgen-deprivation therapy (ADT) in newly diagnosed metastatic, castration-sensitive prostate cancer (mCSPC). Due to significant improvement in overall survival after the first interim analysis, patients in the placebos + ADT arm could switch to AA + P + ADT during an open-label extension. As in other studies where switching is allowed, statistical adjustments are needed to assess the real benefit of new drugs.
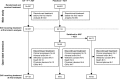
Patients and methods: This was a post hoc analysis to estimate the true survival benefit of AA + P + ADT in patients with newly diagnosed mCSPC by applying statistical adjustments commonly used to adjust for treatment switching.
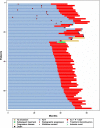
Results: Of 112 patients still receiving placebos + ADT at the first interim analysis, 72 switched to AA + P + ADT during the open-label extension. Final analysis was conducted after median follow-up of 51.8 months. Compared to the placebos + ADT arm, the risk of death in the AA + P + ADT arm was 34% lower [hazard ratio (HR) = 0.663 (95% confidence interval 0.566-0.778)] by unadjusted intent-to-treat analysis, 37% lower [HR = 0.629 (95% confidence interval 0.526-0.753)] by rank preserving structure failure time modeling, and 38% lower [HR = 0.616 (95% confidence interval 0.524-0.724)] by inverse probability of censoring weights.
Conclusions: Analyses adjusting for treatment switching using two different statistical approaches confirm the improved survival benefit of adding AA + P to ADT in patients with newly diagnosed mCSPC.
Trial registration: ClinicalTrials.gov identifier NCT01715285.
Conflict of interest statement
S Feyerabend has nothing to disclose. F. Saad reports grants, personal fees, and non-financial support from Janssen and Sanofi related to the conduct of the study, and grants, personal fees, and non-financial support from Astellas and Bayer unrelated to the submitted work. N.J. Perualila, S. Van Sanden, J. Diels, T. Ito, and P. De Porre are employees of Janssen. S. Van Sanden, T. Ito, and P. De Porre are shareholders of Janssen’s parent company, Johnson & Johnson. K. Fizazi reports financial support for participation in advisory boards for Amgen, AstraZeneca, Astellas, Bayer, Janssen, Sanofi, Orion, and CureVac.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

