Rapid Knee MRI Acquisition and Analysis Techniques for Imaging Osteoarthritis
- PMID: 31755191
- PMCID: PMC7925938
- DOI: 10.1002/jmri.26991
Rapid Knee MRI Acquisition and Analysis Techniques for Imaging Osteoarthritis
Abstract
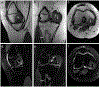
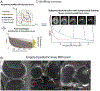
Osteoarthritis (OA) of the knee is a major source of disability that has no known treatment or cure. Morphological and compositional MRI is commonly used for assessing the bone and soft tissues in the knee to enhance the understanding of OA pathophysiology. However, it is challenging to extend these imaging methods and their subsequent analysis techniques to study large population cohorts due to slow and inefficient imaging acquisition and postprocessing tools. This can create a bottleneck in assessing early OA changes and evaluating the responses of novel therapeutics. The purpose of this review article is to highlight recent developments in tools for enhancing the efficiency of knee MRI methods useful to study OA. Advances in efficient MRI data acquisition and reconstruction tools for morphological and compositional imaging, efficient automated image analysis tools, and hardware improvements to further drive efficient imaging are discussed in this review. For each topic, we discuss the current challenges as well as potential future opportunities to alleviate these challenges. LEVEL OF EVIDENCE: 5 TECHNICAL EFFICACY STAGE: 3.
Keywords: compositional imaging; deep learning; morphological imaging; quantitative MRI; rapid MRI; segmentation.
© 2019 International Society for Magnetic Resonance in Medicine.
Figures
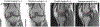









References
Publication types
MeSH terms
Grants and funding
- R00 AR070902/AR/NIAMS NIH HHS/United States
- P50 AR060752/AR/NIAMS NIH HHS/United States
- P41 EB015891/EB/NIBIB NIH HHS/United States
- R01 AR063643/AR/NIAMS NIH HHS/United States
- K12 AR084220/AR/NIAMS NIH HHS/United States
- K99 AR070902/AR/NIAMS NIH HHS/United States
- R61 AR073552/AR/NIAMS NIH HHS/United States
- R01 EB002524/EB/NIBIB NIH HHS/United States
- R00 EB022634/EB/NIBIB NIH HHS/United States
- R01 AR078762/AR/NIAMS NIH HHS/United States
- R01 AR077604/AR/NIAMS NIH HHS/United States
- K12 HD051958/HD/NICHD NIH HHS/United States
- K24 AR062068/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

