Genetic modifiers of risk and age at onset in GBA associated Parkinson's disease and Lewy body dementia
- PMID: 31755958
- PMCID: PMC6935749
- DOI: 10.1093/brain/awz350
Genetic modifiers of risk and age at onset in GBA associated Parkinson's disease and Lewy body dementia
Erratum in
-
Corrigendum.Brain. 2020 Apr 1;143(4):e33. doi: 10.1093/brain/awaa036. Brain. 2020. PMID: 32091085 Free PMC article. No abstract available.
-
Erratum.Brain. 2020 Mar 1;143(3):e24. doi: 10.1093/brain/awaa007. Brain. 2020. PMID: 32333675 Free PMC article. No abstract available.
Abstract
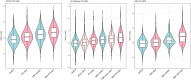
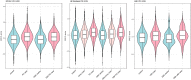
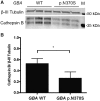
Parkinson's disease is a genetically complex disorder. Multiple genes have been shown to contribute to the risk of Parkinson's disease, and currently 90 independent risk variants have been identified by genome-wide association studies. Thus far, a number of genes (including SNCA, LRRK2, and GBA) have been shown to contain variability across a spectrum of frequency and effect, from rare, highly penetrant variants to common risk alleles with small effect sizes. Variants in GBA, encoding the enzyme glucocerebrosidase, are associated with Lewy body diseases such as Parkinson's disease and Lewy body dementia. These variants, which reduce or abolish enzymatic activity, confer a spectrum of disease risk, from 1.4- to >10-fold. An outstanding question in the field is what other genetic factors that influence GBA-associated risk for disease, and whether these overlap with known Parkinson's disease risk variants. Using multiple, large case-control datasets, totalling 217 165 individuals (22 757 Parkinson's disease cases, 13 431 Parkinson's disease proxy cases, 622 Lewy body dementia cases and 180 355 controls), we identified 1691 Parkinson's disease cases, 81 Lewy body dementia cases, 711 proxy cases and 7624 controls with a GBA variant (p.E326K, p.T369M or p.N370S). We performed a genome-wide association study and analysed the most recent Parkinson's disease-associated genetic risk score to detect genetic influences on GBA risk and age at onset. We attempted to replicate our findings in two independent datasets, including the personal genetics company 23andMe, Inc. and whole-genome sequencing data. Our analysis showed that the overall Parkinson's disease genetic risk score modifies risk for disease and decreases age at onset in carriers of GBA variants. Notably, this effect was consistent across all tested GBA risk variants. Dissecting this signal demonstrated that variants in close proximity to SNCA and CTSB (encoding cathepsin B) are the most significant contributors. Risk variants in the CTSB locus were identified to decrease mRNA expression of CTSB. Additional analyses suggest a possible genetic interaction between GBA and CTSB and GBA p.N370S induced pluripotent cell-derived neurons were shown to have decreased cathepsin B expression compared to controls. These data provide a genetic basis for modification of GBA-associated Parkinson's disease risk and age at onset, although the total contribution of common genetics variants is not large. We further demonstrate that common variability at genes implicated in lysosomal function exerts the largest effect on GBA associated risk for disease. Further, these results have implications for selection of GBA carriers for therapeutic interventions.
Keywords: CTSB; GBA; Parkinson’s disease; SNCA; modifiers.
Published by Oxford University Press on behalf of the Guarantors of Brain 2019. This work is written by US Government employees and is in the public domain in the US.
Figures


References
-
- Abraham G, Qiu Y, Inouye M. FlashPCA2: principal component analysis of Biobank-scale genotype datasets. Bioinformatics 2017; 33: 2776–8. - PubMed
-
- Anheim M, Elbaz A, Lesage S, Durr A, Condroyer C, Viallet F, et al.Penetrance of Parkinson disease in glucocerebrosidase gene mutation carriers. Neurology 2012; 78: 417–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K02 NS080915/NS/NINDS NIH HHS/United States
- G0701075/MRC_/Medical Research Council/United Kingdom
- G1100643/MRC_/Medical Research Council/United Kingdom
- Z01 ES101986/ImNIH/Intramural NIH HHS/United States
- K23 AG059891/AG/NIA NIH HHS/United States
- P50 NS038377/NS/NINDS NIH HHS/United States
- MR/N026004/1/MRC_/Medical Research Council/United Kingdom
- J-1101/PUK_/Parkinson's UK/United Kingdom
- G0901254/MRC_/Medical Research Council/United Kingdom
- G0700943/MRC_/Medical Research Council/United Kingdom
- P30 AG066507/AG/NIA NIH HHS/United States
- MR/L501542/1/MRC_/Medical Research Council/United Kingdom
- G1100540/MRC_/Medical Research Council/United Kingdom
- Z01 AG000949/ImNIH/Intramural NIH HHS/United States
- MR/K01417X/1/MRC_/Medical Research Council/United Kingdom
- G-0907/PUK_/Parkinson's UK/United Kingdom
- P50 AG005146/AG/NIA NIH HHS/United States
- H-1703/PUK_/Parkinson's UK/United Kingdom
- ZIA NS003154/ImNIH/Intramural NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- U19 AG033655/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

