Tauroursodeoxycholate-Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives
- PMID: 31757001
- PMCID: PMC6952947
- DOI: 10.3390/cells8121471
Tauroursodeoxycholate-Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives
Abstract
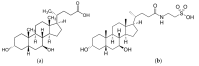
Tauroursodeoxycholic acid (TUDCA) is a naturally occurring hydrophilic bile acid that has been used for centuries in Chinese medicine. Chemically, TUDCA is a taurine conjugate of ursodeoxycholic acid (UDCA), which in contemporary pharmacology is approved by Food and Drug Administration (FDA) for treatment of primary biliary cholangitis. Interestingly, numerous recent studies demonstrate that mechanisms of TUDCA functioning extend beyond hepatobiliary disorders. Thus, TUDCA has been demonstrated to display potential therapeutic benefits in various models of many diseases such as diabetes, obesity, and neurodegenerative diseases, mostly due to its cytoprotective effect. The mechanisms underlying this cytoprotective activity have been mainly attributed to alleviation of endoplasmic reticulum (ER) stress and stabilization of the unfolded protein response (UPR), which contributed to naming TUDCA as a chemical chaperone. Apart from that, TUDCA has also been found to reduce oxidative stress, suppress apoptosis, and decrease inflammation in many in-vitro and in-vivo models of various diseases. The latest research suggests that TUDCA can also play a role as an epigenetic modulator and act as therapeutic agent in certain types of cancer. Nevertheless, despite the massive amount of evidence demonstrating positive effects of TUDCA in pre-clinical studies, there are certain limitations restraining its wide use in patients. Here, molecular and cellular modes of action of TUDCA are described and therapeutic opportunities and limitations of this bile acid are discussed.
Keywords: ER stress; TUDCA; apoptosis; bile acids; cytoprotection; inflammation.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

