Cytosolic Acidification Is the First Transduction Signal of Lactoferrin-induced Regulated Cell Death Pathway
- PMID: 31757076
- PMCID: PMC6928705
- DOI: 10.3390/ijms20235838
Cytosolic Acidification Is the First Transduction Signal of Lactoferrin-induced Regulated Cell Death Pathway
Abstract
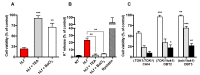
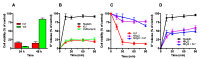
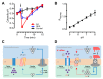
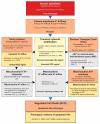
In yeast, we reported the critical role of K+-efflux for the progress of the regulated cell death (RCD) induced by human lactoferrin (hLf), an antimicrobial protein of the innate immune system that blocks Pma1p H+-ATPase. In the present study, the K+ channel Tok1p was identified as the K+ channel-mediating K+-efflux, as indicated by the protective effect of extracellular K+ (30 mM), K+-channel blockers, and the greater hLf-resistance of TOK1-disrupted strains. K+-depletion was necessary but not sufficient to induce RCD as inferred from the effects of valinomycin, NH4Cl or nigericin which released a percentage of K+ similar to that released by lactoferrin without affecting cell viability. Cytosolic pH of hLf-treated cells decreased transiently (0.3 pH units) and its inhibition prevented the RCD process, indicating that cytosolic acidification was a necessary and sufficient triggering signal. The blocking effect of lactoferrin on Pma1p H+-ATPase caused a transitory decrease of cytosolic pH, and the subsequent membrane depolarization activated the voltage-gated K+ channel, Tok1p, allowing an electrogenic K+-efflux. These ionic events, cytosolic accumulation of H+ followed by K+-efflux, constituted the initiating signals of this mitochondria-mediated cell death. These findings suggest, for the first time, the existence of an ionic signaling pathway in RCD.
Keywords: Candida albicans; apoptosis-like; cell signaling pathway; cytosolic acidification; lactoferrin; potassium efflux; regulated cell death.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

