Nucleoplasmic Reticulum Formation in Human Endometrial Cells is Steroid Hormone Responsive and Recruits Nascent Components
- PMID: 31757079
- PMCID: PMC6929123
- DOI: 10.3390/ijms20235839
Nucleoplasmic Reticulum Formation in Human Endometrial Cells is Steroid Hormone Responsive and Recruits Nascent Components
Abstract
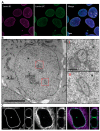
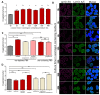
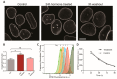
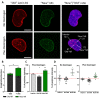
The nuclei of cells may exhibit invaginations of the nuclear envelope under a variety of conditions. These invaginations form a branched network termed the nucleoplasmic reticulum (NR), which may be found in cells in pathological and physiological conditions. While an extensive NR is a hallmark of cellular senescence and shows associations with some cancers, very little is known about the formation of NR in physiological conditions, despite the presence of extensive nuclear invaginations in some cell types such as endometrial cells. Here we show that in these cells the NR is formed in response to reproductive hormones. We demonstrate that oestrogen and progesterone are sufficient to induce NR formation and that this process is reversible without cell division upon removal of the hormonal stimulus. Nascent lamins and phospholipids are incorporated into the invaginations suggesting that there is a dedicated machinery for its formation. The induction of NR in endometrial cells offers a new model to study NR formation and function in physiological conditions.
Keywords: nuclear architecture; nucleoplasmic reticulum; reproductive cycle.
Conflict of interest statement
Authors declare no conflicts of interest with the contents of this article.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

