Midbrain microglia mediate a specific immunosuppressive response under inflammatory conditions
- PMID: 31757220
- PMCID: PMC6874825
- DOI: 10.1186/s12974-019-1628-8
Midbrain microglia mediate a specific immunosuppressive response under inflammatory conditions
Abstract
Background: Inflammation is a critical process for the progression of neuronal death in neurodegenerative disorders. Microglia play a central role in neuroinflammation and may affect neuron vulnerability. Next generation sequencing has shown the molecular heterogeneity of microglial cells; however, the variability in their response to pathological inputs remains unknown.
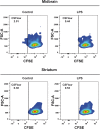
Methods: To determine the effect of an inflammatory stimulus on microglial cells, lipopolysaccharide (LPS) was administered peripherally to mice and the inflammatory status of the cortex, hippocampus, midbrain, and striatum was assessed. Microglial activation and interaction with the immune system were analyzed in single cell suspensions obtained from the different brain regions by fluorescence-activated cell sorting, next generation RNA sequencing, real-time PCR, and immunohistochemical techniques. Antigen-presenting properties of microglia were evaluated by the ability of isolated cells to induce a clonal expansion of CD4+ T cells purified from OT-II transgenic mice.
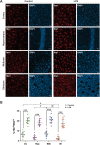
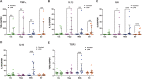
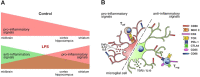
Results: Under steady-state conditions, the midbrain presented a high immune-alert state characterized by the presence of two unique microglial subpopulations, one expressing the major histocompatibility complex class II (MHC-II) and acting as antigen-presenting cells and another expressing the toll-like receptor 4 (TLR4), and by the presence of a higher proportion of infiltrating CD4+ T cells. This state was not detected in the cortex, hippocampus, or striatum. Systemic LPS administration induced a general increase in classic pro-inflammatory cytokines, in co-inhibitory programmed death ligand 1 (PD-L1), and in cytotoxic T lymphocyte antigen 4 (CTLA-4) receptors, as well as a decrease in infiltrating effector T cells in all brain regions. Interestingly, a specific immune-suppressive response was observed in the midbrain which was characterized by the downregulation of MHC-II microglial expression, the upregulation of the anti-inflammatory cytokines IL10 and TGFβ, and the increase in infiltrating regulatory T cells.
Conclusions: These data show that the midbrain presents a high immune-alert state under steady-state conditions that elicits a specific immune-suppressive response when exposed to an inflammatory stimulus. This specific inflammatory tone and response may have an impact in neuronal viability.
Keywords: Antigen-presenting cells; Innate immunity; LPS; MHC-II; Microglia; Midbrain; TGFβ; Treg.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

