Live Respiratory Syncytial Virus Attenuated by M2-2 Deletion and Stabilized Temperature Sensitivity Mutation 1030s Is a Promising Vaccine Candidate in Children
- PMID: 31758177
- PMCID: PMC6996856
- DOI: 10.1093/infdis/jiz603
Live Respiratory Syncytial Virus Attenuated by M2-2 Deletion and Stabilized Temperature Sensitivity Mutation 1030s Is a Promising Vaccine Candidate in Children
Abstract
Background: The safety and immunogenicity of live respiratory syncytial virus (RSV) candidate vaccine, LID/ΔM2-2/1030s, with deletion of RSV ribonucleic acid synthesis regulatory protein M2-2 and genetically stabilized temperature-sensitivity mutation 1030s in the RSV polymerase protein was evaluated in RSV-seronegative children.
Methods: Respiratory syncytial virus-seronegative children ages 6-24 months received 1 intranasal dose of 105 plaque-forming units (PFU) of LID/ΔM2-2/1030s (n = 21) or placebo (n = 11). The RSV serum antibodies, vaccine shedding, and reactogenicity were assessed. During the following RSV season, medically attended acute respiratory illness (MAARI) and pre- and postsurveillance serum antibody titers were monitored.
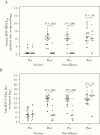
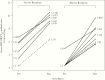
Results: Eighty-five percent of vaccinees shed LID/ΔM2-2/1030s vaccine (median peak nasal wash titers: 3.1 log10 PFU/mL by immunoplaque assay; 5.1 log10 copies/mL by reverse-transcription quantitative polymerase chain reaction) and had ≥4-fold rise in serum-neutralizing antibodies. Respiratory symptoms and fever were common (60% vaccinees and 27% placebo recipients). One vaccinee had grade 2 wheezing with rhinovirus but without concurrent LID/ΔM2-2/1030s shedding. Five of 19 vaccinees had ≥4-fold increases in antibody titers postsurveillance without RSV-MAARI, indicating anamnestic responses without significant illness after infection with community-acquired RSV.
Conclusions: LID/ΔM2-2/1030s had excellent infectivity without evidence of genetic instability, induced durable immunity, and primed for anamnestic antibody responses, making it an attractive candidate for further evaluation.
Keywords: RNA regulatory protein M2-2; live-attenuated viral vaccine; neutralizing antibodies; pediatric RSV vaccine; respiratory syncytial virus (RSV).
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- Karron RA, Black RE. Determining the burden of respiratory syncytial virus disease: the known and the unknown. Lancet 2017; 390:917–8. - PubMed
-
- Geoghegan S, Erviti A, Caballero MT, et al. . Mortality due to respiratory syncytial virus. burden and risk factors. Am J Respir Crit Care Med 2017; 195:96–103. - PubMed
-
- Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr 2000; 137:865–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

