Effects of a nutrition intervention on acute and late bowel symptoms and health-related quality of life up to 24 months post radiotherapy in patients with prostate cancer: a multicentre randomised controlled trial
- PMID: 31758324
- PMCID: PMC7256032
- DOI: 10.1007/s00520-019-05182-5
Effects of a nutrition intervention on acute and late bowel symptoms and health-related quality of life up to 24 months post radiotherapy in patients with prostate cancer: a multicentre randomised controlled trial
Abstract
Purpose: Radiotherapy to the prostate gland and pelvic lymph nodes may cause acute and late bowel symptoms and diminish quality of life. The aim was to study the effects of a nutrition intervention on bowel symptoms and health-related quality of life, compared with standard care.
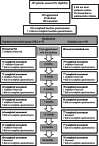
Methods: Patients were randomised to a nutrition intervention (n = 92) aiming to replace insoluble fibres with soluble and reduce intake of lactose, or a standard care group (n = 88) who were recommended to maintain their habitual diet. Bowel symptoms, health-related quality of life and intake of fibre and lactose-containing foods were assessed up to 24 months after radiotherapy completion. Multiple linear regression was used to analyse the effects of the nutrition intervention on bowel symptoms during the acute (up to 2 months post radiotherapy) and the late (7 to 24 months post radiotherapy) phase.
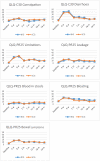
Results: Most symptoms and functioning worsened during the acute phase, and improved during the late phase in both the intervention and standard care groups. The nutrition intervention was associated with less blood in stools (p = 0.047), flatulence (p = 0.014) and increased loss of appetite (p = 0.018) during the acute phase, and more bloated abdomen in the late phase (p = 0.029). However, these associations were clinically trivial or small.
Conclusions: The effect of the nutrition intervention related to dietary fibre and lactose on bowel symptoms from pelvic RT was small and inconclusive, although some minor and transient improvements were observed. The results do not support routine nutrition intervention of this type to reduce adverse effects from pelvic radiotherapy.
Keywords: Bowel symptoms; Nutrition intervention; Prostate cancer; Radiotherapy.
Figures

Similar articles
-
Effects of a dietary intervention on acute gastrointestinal side effects and other aspects of health-related quality of life: a randomized controlled trial in prostate cancer patients undergoing radiotherapy.Radiother Oncol. 2012 Jun;103(3):333-40. doi: 10.1016/j.radonc.2012.04.006. Epub 2012 May 24. Radiother Oncol. 2012. PMID: 22633817 Clinical Trial.
-
Effects of a dietary intervention on gastrointestinal symptoms after prostate cancer radiotherapy: long-term results from a randomized controlled trial.Radiother Oncol. 2014 Nov;113(2):240-7. doi: 10.1016/j.radonc.2014.11.025. Epub 2014 Nov 29. Radiother Oncol. 2014. PMID: 25467005 Clinical Trial.
-
Experiences of a nutrition intervention-A qualitative study within a randomised controlled trial in men undergoing radiotherapy for prostate cancer.Nutr Diet. 2020 Apr;77(2):223-230. doi: 10.1111/1747-0080.12564. Epub 2019 Jun 27. Nutr Diet. 2020. PMID: 31243870 Clinical Trial.
-
Quality of life study in prostate cancer patients treated with three-dimensional conformal radiation therapy: comparing late bowel and bladder quality of life symptoms to that of the normal population.Int J Radiat Oncol Biol Phys. 2001 Jan 1;49(1):51-9. doi: 10.1016/s0360-3016(00)01365-1. Int J Radiat Oncol Biol Phys. 2001. PMID: 11163497 Review.
-
Nutritional strategies to prevent gastrointestinal toxicity during pelvic radiotherapy.Proc Nutr Soc. 2018 Nov;77(4):357-368. doi: 10.1017/S0029665118000101. Epub 2018 Apr 2. Proc Nutr Soc. 2018. PMID: 29607792 Review.
Cited by
-
Nutrition as prevention for improved cancer health outcomes: a systematic literature review.JNCI Cancer Spectr. 2023 May 2;7(3):pkad035. doi: 10.1093/jncics/pkad035. JNCI Cancer Spectr. 2023. PMID: 37212631 Free PMC article.
-
Nutritional counseling was insufficient to maintain dietary intake and nutritional status in head and neck cancer patients undergoing radiotherapy: A historical control study for future intervention in China.Asia Pac J Oncol Nurs. 2022 Feb 12;9(4):190-196. doi: 10.1016/j.apjon.2022.01.013. eCollection 2022 Apr. Asia Pac J Oncol Nurs. 2022. PMID: 35571622 Free PMC article.
-
Dietary Intervention Improves Gastrointestinal Symptoms after Treatment of Cancer in the Pelvic Organs.J Clin Med. 2023 Jul 19;12(14):4766. doi: 10.3390/jcm12144766. J Clin Med. 2023. PMID: 37510881 Free PMC article.
-
The effect of nutritional interventions involving dietary counselling on gastrointestinal toxicities in adults receiving pelvic radiotherapy - A systematic review.J Med Radiat Sci. 2021 Dec;68(4):453-464. doi: 10.1002/jmrs.531. Epub 2021 Jul 20. J Med Radiat Sci. 2021. PMID: 34288532 Free PMC article.
-
Assessing Impact of Nutrition Care by Registered Dietitian Nutritionists on Patient Medical and Treatment Outcomes in Outpatient Cancer Clinics: A Cohort Feasibility Study.Nutr Cancer. 2023;75(3):923-936. doi: 10.1080/01635581.2023.2170431. Epub 2023 Jan 24. Nutr Cancer. 2023. PMID: 36691979 Free PMC article.
References
-
- Khalid U, McGough C, Hackett C, Blake P, Harrington KJ, Khoo VS, Tait D, Norman AR, Andreyev HJ. A modified inflammatory bowel disease questionnaire and the Vaizey Incontinence questionnaire are more sensitive measures of acute gastrointestinal toxicity during pelvic radiotherapy than RTOG grading. Int J Radiat Oncol Biol Phys. 2006;64(5):1432–1441. doi: 10.1016/j.ijrobp.2005.10.007. - DOI - PubMed
-
- Lawrie TA, Green JT, Beresford M, Wedlake L, Burden S, Davidson SE, Lal S, Henson CC, Andreyev HJN. Interventions to reduce acute and late adverse gastrointestinal effects of pelvic radiotherapy for primary pelvic cancers. Cochrane Database Syst Rev. 2018;1:Cd012529. doi: 10.1002/14651858.CD012529.pub2. - DOI - PMC - PubMed
-
- Dearnaley DP, Khoo VS, Norman AR, Meyer L, Nahum A, Tait D, Yarnold J, Horwich A. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet (London, England) 1999;353(9149):267–272. doi: 10.1016/s0140-6736(98)05180-0. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

