Lurasidone compared to other atypical antipsychotic monotherapies for adolescent schizophrenia: a systematic literature review and network meta-analysis
- PMID: 31758359
- PMCID: PMC7497364
- DOI: 10.1007/s00787-019-01425-2
Lurasidone compared to other atypical antipsychotic monotherapies for adolescent schizophrenia: a systematic literature review and network meta-analysis
Abstract
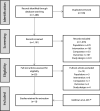
This network meta-analysis assessed the efficacy and tolerability of lurasidone versus other oral atypical antipsychotic monotherapies in adolescent schizophrenia. A systematic literature review identified 13 randomized controlled trials of antipsychotics in adolescents with schizophrenia-spectrum disorders. A Bayesian network meta-analysis compared lurasidone to aripiprazole, asenapine, clozapine, olanzapine, paliperidone extended-release (ER), quetiapine, risperidone, and ziprasidone. Outcomes included Positive and Negative Syndrome Scale (PANSS), Clinical Global Impressions-Severity (CGI-S), weight gain, all-cause discontinuation, extrapyramidal symptoms (EPS), and akathisia. Results were reported as median differences for continuous outcomes and odds ratios (ORs) for binary outcomes, along with 95% credible intervals (95% CrI). Lurasidone was significantly more efficacious than placebo on the PANSS (- 7.95, 95% CrI - 11.76 to - 4.16) and CGI-S (- 0.44, 95% CrI - 0.67 to - 0.22) scores. Lurasidone was associated with similar weight gain to placebo and statistically significantly less weight gain versus olanzapine (- 3.62 kg, 95% CrI - 4.84 kg to - 2.41 kg), quetiapine (- 2.13 kg, 95% CrI - 3.20 kg to - 1.08 kg), risperidone (- 1.16 kg, 95% CrI - 2.14 kg to - 0.17 kg), asenapine (- 0.98 kg, 95% CrI - 1.71 kg to - 0.24 kg), and paliperidone ER (- 0.85 kg, 95% CrI - 1.57 kg to - 0.14 kg). The odds of all-cause discontinuation were significantly lower for lurasidone than aripiprazole (OR = 0.28, 95% CrI 0.10-0.76) and paliperidone ER (OR = 0.25, 95% CrI 0.08-0.81) and comparable to other antipsychotics. Rates of EPS and akathisia were similar for lurasidone and other atypical antipsychotics. In this network meta-analysis of atypical antipsychotics in adolescent schizophrenia, lurasidone was associated with similar efficacy, less weight gain, and lower risk of all-cause discontinuation compared to other oral atypical antipsychotics.
Keywords: Adolescent; Body weight changes; Lurasidone; Network meta-analysis; Schizophrenia.
Conflict of interest statement
DNg-Mak and A Loebel are employees of Sunovion Pharmaceuticals Inc. E Finn and A Byrne were consultants paid by Sunovion Pharmaceuticals Inc. to conduct the study analysis. C Arango has been a consultant to or has received honoraria or grants from Acadia, Angellini, Gedeon Richter, Janssen Cilag, Lundbeck, Merck, Otsuka, Roche, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion and Takeda.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

