IL-1-dependent electrophysiological changes and cardiac neural remodeling in a mouse model of Kawasaki disease vasculitis
- PMID: 31758701
- PMCID: PMC7008220
- DOI: 10.1111/cei.13401
IL-1-dependent electrophysiological changes and cardiac neural remodeling in a mouse model of Kawasaki disease vasculitis
Abstract
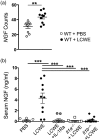
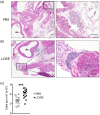
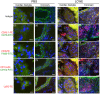
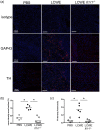
Kawasaki disease (KD) is the leading cause of acquired heart disease in children. In addition to coronary artery abnormalities, aneurysms and myocarditis, acute KD is also associated with echocardiogram (ECG) abnormalities in 40-80% of patients. Here, we show that these ECG changes are recapitulated in the Lactobacillus casei cell wall extract (LCWE)-induced KD vasculitis mouse model. LCWE-injected mice developed elevated heart rate and decreased R wave amplitude, with significant differences in prolonged ventricular repolarization. LCWE-injected mice developed cardiac ganglion inflammation, that may affect the impulse-conducting system in the myocardium. Furthermore, serum nerve growth factor (NGF) was significantly elevated in LCWE-injected mice, similar to children with KD vasculitis, associated with increased neural remodeling of the myocardium. ECG abnormalities were prevented by blocking interleukin (IL)-1 signaling with anakinra, and the increase in serum NGF and cardiac neural remodeling were similarly blocked in Il1r1-/- mice and in wild-type mice treated with anakinra. Thus, similar to clinical KD, the LCWE-induced KD vasculitis mouse model also exhibits electrophysiological abnormalities and cardiac neuronal remodeling, and these changes can be prevented by blocking IL-1 signaling. These data support the acceleration of anti-IL-1 therapy trials to benefit KD patients.
© 2019 British Society for Immunology.
Conflict of interest statement
None to declare.
Figures

References
-
- Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 1974; 54:271–6. - PubMed
-
- McCrindle BW, Rowley AH, Newburger JW et al, on behalf of the American Heart Association Rheumatic Fever, Endocarditis, Kawasaki Disease, Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia ; Council on Epidemiology and Prevention . Diagnosis, treatment, and long‐term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017; 135:e927–99. - PubMed
-
- Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol 2016; 67:1738–49. - PubMed
-
- Rowley AH. Finding the cause of Kawasaki disease: a pediatric infectious diseases research priority. J Infectious Dis 2006; 194:1635–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

