Achieving the composite end-point of glycated hemoglobin <7.0% without weight gain or hypoglycemia with once-weekly dulaglutide in Chinese patients with type 2 diabetes: A post-hoc analysis
- PMID: 31758850
- PMCID: PMC7232276
- DOI: 10.1111/jdi.13187
Achieving the composite end-point of glycated hemoglobin <7.0% without weight gain or hypoglycemia with once-weekly dulaglutide in Chinese patients with type 2 diabetes: A post-hoc analysis
Abstract
Aims/introduction: To assess the effect of dulaglutide (DU) 1.5/0.75 mg in comparison with glimepiride (GLIM) or insulin glargine (GLAR) on the composite end-point in Chinese type 2 diabetes patients.
Materials and methods: Post-hoc analyses of two randomized phase III trials (NCT01644500 and NCT01648582) were carried out using Fisher's exact test. The primary composite end-point was the number of patients reaching glycated hemoglobin (HbA1c) <7.0%, without weight gain and hypoglycemia. Secondary composite end-points included the number of patients reaching HbA1c <7.0% without weight gain and HbA1c <7.0% without hypoglycemia.
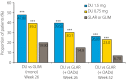
Results: Data of 1,147 Chinese type 2 diabetes patients were analyzed (NCT01644500 = 556; NCT01648582 = 591). In each analyzed trial, 40-48% of patients received DU (1.5 mg), 30-39% of patients received DU (0.75 mg) and 15-20% of patients on active comparators (GLIM/GLAR) reached the primary composite end-point at week 26 (P < 0.001 for DU vs GLIM/GLAR). At 52 weeks, 26% of patients that received DU (1.5 mg), 23% of patients that received DU (0.75 mg) and 7% of patients that received GLAR attained the primary composite end-point (P < 0.001 for DU vs GLAR). A similar trend of results was found for secondary composite end-points.
Conclusions: Dulaglutide is found to be an effective therapeutic alternative for Chinese type 2 diabetes patients. Compared with GLIM/GLAR, significantly greater proportions of patients on DU attained the HbA1c target of <7.0% without weight gain or hypoglycemia.
Keywords: Composite end-point; Dulaglutide; Type 2 diabetes.
© 2019 Lilly Suzhou Pharmaceutical Co. Ltd. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
BZ and JNH are employees of Eli Lilly and Company. LQG was an employee of Eli Lilly and Company at the time of manuscript preparation. The other authors declare no conflict if interest.
Figures

References
-
- Guo XH, Yuan L, Lou QQ, et al A nationwide survey of diabetes education, self‐management and glycemic control in patients with type 2 diabetes in China. Chin Med J (Engl) 2012; 125: 4175–4180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

