High-Dose Radiation Increases Notch1 in Tumor Vasculature
- PMID: 31759078
- PMCID: PMC8048139
- DOI: 10.1016/j.ijrobp.2019.11.010
High-Dose Radiation Increases Notch1 in Tumor Vasculature
Abstract
Purpose: The aim of this study is to characterize the effects of high-dose radiation therapy (HDRT) on Notch signaling components of the tumor vasculature.
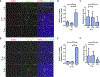
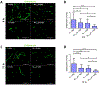
Methods and materials: Human umbilical vein endothelial cells monolayers were exposed to different single fraction doses of irradiation; ribonucleic acid RNA was isolated and polymerase chain reaction was performed for Notch signaling components. The vascular response to radiation therapy was examined in a xenograft model of neuroblastoma. Tumors were treated with 0 Gy, 2 Gy, and 12 Gy single fraction doses and analyzed by double immunofluorescence staining for Notch1, Notch ligands Jagged1 and Dll4, and the endothelial cell (EC) marker endomucin. To assess the role of Notch in vivo, NGP xenograft tumors expressing Fc or Notch1-1-24-decoy (a novel Notch inhibitor) were treated with 0 Gy and 12 Gy. Immunofluorescence staining for endomucin and endomucin/αSMA was performed to analyze the effect of combination treatment on tumor EC and endothelial-to-mesenchymal-transition (EndMT), respectively.
Results: In human umbilical vein endothelial cells monolayers doses ≥8 Gy increased expression of NOTCH1, JAG1, and Notch target genes HEY1 and HEY2 as early as 6 hours after irradiation. In vivo, 12 Gy significantly increased Notch1 and Jagged1 in tumor ECs compared with 0 Gy or 2 Gy after 72 hours. Combining HDRT with Notch inhibition using the Notch1-1-24-decoy resulted in a greater loss of EC coverage of tumor vessels than HDRT alone at 6 hours and 72 hours post treatment. Notch inhibition reduced EndMT induced by HDRT, as indicated by diminished αSMA staining in ECs.
Conclusions: HDRT induced Notch1 expression and increased Notch1 signaling in the endothelial component of tumor vasculature, which was not observed with lower doses. This increase in Notch1 activation might protect tumor vessels from HDRT induced damage and regulate EndMT process.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Lo SS, Fakiris AJ, Chang EL, et al. Stereotactic body radiation therapy: A novel treatment modality. Nat Rev Clin Oncol 2010;7:44–54. - PubMed
-
- Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003; 300:1155–1159. - PubMed
-
- Song CW, Kim MS, Cho LC, et al. Radiobiological basis of SBRT and SRS. Int J Clin Oncol 2014;19:570–578. - PubMed
-
- Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol 2008;18:240–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

