Treatment of chronic hepatitis C in patients with chronic kidney disease with Sofosbuvir-basead regimes
- PMID: 31760038
- PMCID: PMC9392030
- DOI: 10.1016/j.bjid.2019.10.011
Treatment of chronic hepatitis C in patients with chronic kidney disease with Sofosbuvir-basead regimes
Abstract
Background: To analyze the effectiveness and the safety of Sofosbuvir-based regimens to treat patients with chronic hepatitis C virus (HCV) infection and chronic kidney disease (CKD).
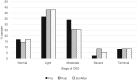
Methods: A retrospective, observational study in patients with chronic HCV infection and CKD treated with Sofosbuvir-based regimens was performed. Liver fibrosis, comorbidities, HCV genotype and sustained virological resposnse (SVR) at 12th week post-treatment were evaluated. Kidney function was accessed by serum creatinine and glomerular filtration rate (GFR). The assumed level of significance was 5 %.
Results: Thirty-five patients were treated. The mean age was 52.1±10.9 years, 19 (54.3 %) were women, 32 (91.4 %) were already kidney transplanted and 3 (8.6 %) were on hemodialysis. The SVR by intention to treat was 88.6 %. The mean GFR was 65.8±28.6 and 63.7±28.3ml/min pre- and post-treatment respectively (p>0.05). Treatment was interrupted in 1 (2.85 %) patient due to anemia and in 2 (5.7 %) due to loss of kidney function.
Conclusion: Sofosbuvir-based regimens are effective to treat HCV in patients with CKD. In patients with mild CKD this type of therapy seems to be safe.
Keywords: Daclatasvir; Direct-acting antiviral; Hemodialysis; Kidney transplantion; Sofosbuvir.
Copyright © 2020 Sociedade Brasileira de Infectologia. Published by Elsevier España, S.L.U. All rights reserved.
Figures
References
-
- Jadoul M., Bieber B.A., Martin P., Akiba T., Nwankwo C., Arduino J.M., Goodkin D.A., Pisoni R.L. Prevalence, incidence, and risk factors for hepatitis C virus infection in hemodialysis patients. Kidney Int. 2019;4:939–947. - PubMed
-
- Sesso R.C., Lopes A.A., Thomé F.S., Lugon J.R., Martins C.T. Brazilian Chronic Dialysis Census 2014. J Bras Nefrol. 2016;38:54–61. - PubMed
-
- Gordon C.E., Berenguer M.C., Doss W., Fabrizi F., Izopet J., Jha V., et al. Prevention, diagnosis, evaluation, and treatment of hepatitis C virus infection in chronic kidney disease: synopsis of the kidney disease: Improving Global Outcomes 2018 Clinical Practice Guideline. Ann Intern Med. 2019;171:496–504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical