Neurovascular coupling preserved in a chronic mouse model of Alzheimer's disease: Methodology is critical
- PMID: 31760864
- PMCID: PMC7585931
- DOI: 10.1177/0271678X19890830
Neurovascular coupling preserved in a chronic mouse model of Alzheimer's disease: Methodology is critical
Abstract
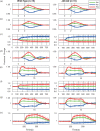
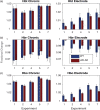
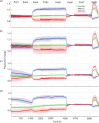
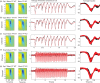
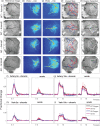
Impaired neurovascular coupling has been suggested as an early pathogenic factor in Alzheimer's disease (AD), which could serve as an early biomarker of cerebral pathology. We have established an anaesthetic regime to allow repeated measurements of neurovascular function over three months in the J20 mouse model of AD (J20-AD) and wild-type (WT) controls. Animals were 9-12 months old at the start of the experiment. Mice were chronically prepared with a cranial window through which 2-Dimensional optical imaging spectroscopy (2D-OIS) was used to generate functional maps of the cerebral blood volume and saturation changes evoked by whisker stimulation and vascular reactivity challenges. Unexpectedly, the hemodynamic responses were largely preserved in the J20-AD group. This result failed to confirm previous investigations using the J20-AD model. However, a final acute electrophysiology and 2D-OIS experiment was performed to measure both neural and hemodynamic responses concurrently. In this experiment, previously reported deficits in neurovascular coupling in the J20-AD model were observed. This suggests that J20-AD mice may be more susceptible to the physiologically stressing conditions of an acute experimental procedure compared to WT animals. These results therefore highlight the importance of experimental procedure when determining the characteristics of animal models of human disease.
Keywords: Barrel cortex; J20; blood flow; electrophysiology; optical imaging.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

