Multiday maintenance of extracorporeal lungs using cross-circulation with conscious swine
- PMID: 31761338
- PMCID: PMC7094131
- DOI: 10.1016/j.jtcvs.2019.09.121
Multiday maintenance of extracorporeal lungs using cross-circulation with conscious swine
Abstract
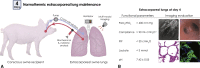
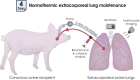
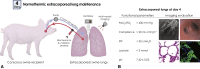
Objectives: Lung remains the least-utilized solid organ for transplantation. Efforts to recover donor lungs with reversible injuries using ex vivo perfusion systems are limited to <24 hours of support. Here, we demonstrate the feasibility of extending normothermic extracorporeal lung support to 4 days using cross-circulation with conscious swine.
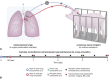
Methods: A swine behavioral training program and custom enclosure were developed to enable multiday cross-circulation between extracorporeal lungs and recipient swine. Lungs were ventilated and perfused in a normothermic chamber for 4 days. Longitudinal analyses of extracorporeal lungs (ie, functional assessments, multiscale imaging, cytokine quantification, and cellular assays) and recipient swine (eg, vital signs and blood and tissue analyses) were performed.
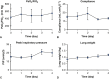
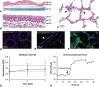
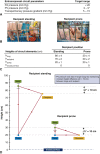
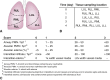
Results: Throughout 4 days of normothermic support, extracorporeal lung function was maintained (arterial oxygen tension/inspired oxygen fraction >400 mm Hg; compliance >20 mL/cm H2O), and recipient swine were hemodynamically stable (lactate <3 mmol/L; pH, 7.42 ± 0.05). Radiography revealed well-aerated lower lobes and consolidation in upper lobes of extracorporeal lungs, and bronchoscopy showed healthy airways without edema or secretions. In bronchoalveolar lavage fluid, granulocyte-macrophage colony-stimulating factor, interleukin (IL) 4, IL-6, and IL-10 levels increased less than 6-fold, whereas interferon gamma, IL-1α, IL-1β, IL-1ra, IL-2, IL-8, IL-12, IL-18, and tumor necrosis factor alpha levels decreased from baseline to day 4. Histologic evaluations confirmed an intact blood-gas barrier and outstanding preservation of airway and alveolar architecture. Cellular viability and metabolism in extracorporeal lungs were confirmed after 4 days.
Conclusions: We demonstrate feasibility of normothermic maintenance of extracorporeal lungs for 4 days by cross-circulation with conscious swine. Cross-circulation approaches could support the recovery of damaged lungs and enable organ bioengineering to improve transplant outcomes.
Keywords: acute lung injury; airway lavage; alveolar recruitment; bronchoalveolar lavage fluid; chimerism; cross-circulation; ex vivo lung perfusion; extracorporeal membrane oxygenation; infrared thermography; lung bioengineering; lung transplantation; medical thermography; normothermic organ perfusion; organ shortage; regenerative medicine; swine model; tissue engineering; transplantation; whole organ bioreactor.
Copyright © 2019 The American Association for Thoracic Surgery. Published by Elsevier Inc. All rights reserved.
Figures












Comment in
-
Commentary: Cross circulation comes full circle (via lung transplantation).J Thorac Cardiovasc Surg. 2020 Apr;159(4):1654-1655. doi: 10.1016/j.jtcvs.2019.09.045. Epub 2019 Sep 24. J Thorac Cardiovasc Surg. 2020. PMID: 31676106 No abstract available.
-
Commentary: Development of a new concept is achieved only step-by-step.J Thorac Cardiovasc Surg. 2020 Apr;159(4):1656-1657. doi: 10.1016/j.jtcvs.2019.09.109. Epub 2019 Oct 1. J Thorac Cardiovasc Surg. 2020. PMID: 31677886 No abstract available.
-
Discussion.J Thorac Cardiovasc Surg. 2020 Apr;159(4):1652-1653. doi: 10.1016/j.jtcvs.2019.09.122. Epub 2019 Nov 22. J Thorac Cardiovasc Surg. 2020. PMID: 31761351 No abstract available.
References
-
- Ware L.B., Wang Y., Fang X., Warnock M., Sakuma T., Hall T.S. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet. 2002;360:619–620. - PubMed
-
- Kugler C., Gottlieb J., Warnecke G., Schwarz A., Weissenborn K., Barg-Hock H. Health-related quality of life after solid organ transplantation: a prospective, multiorgan cohort study. Transplant J. 2013;96:316–323. - PubMed
E-References
-
- O'Neill J.D., Guenthart B.A., Kim J., Chicotka S., Queen D., Fung K. Cross-circulation for extracorporeal support and recovery of the lung. Nat Biomed Eng. 2017;1:0037.
-
- Roman M., Gjorgjimajkoska O., Neil D., Nair S., Colah S., Parmar J. Comparison between cellular and acellular perfusates for ex vivo lung perfusion in a porcine model. J Heart Lung Transplant. 2015;34:978–987. - PubMed
-
- Jung K., Renukaradhya G.J., Alekseev K.P., Fang Y., Tang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J Gen Virol. 2009;90(Pt 11):2713–2723. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

