Atypical antipsychotic use during pregnancy and birth defect risk: National Birth Defects Prevention Study, 1997-2011
- PMID: 31761471
- PMCID: PMC7036025
- DOI: 10.1016/j.schres.2019.11.019
Atypical antipsychotic use during pregnancy and birth defect risk: National Birth Defects Prevention Study, 1997-2011
Abstract
Purpose: To examine the prevalence of, and factors associated with, atypical antipsychotic use among U.S. pregnant women, and potential associations between early pregnancy atypical antipsychotic use and risk for 14 birth defects.
Methods: We analyzed data from the National Birth Defects Prevention Study (1997-2011), a U.S. population-based case-control study examining risk factors for major structural birth defects.
Results: Atypical antipsychotic use during pregnancy was more common among women with pre-pregnancy obesity, and women who reported illicit drug use before and during pregnancy, smoking during pregnancy, alcohol use during pregnancy, or use of other psychiatric medications during pregnancy. We observed elevated associations (defined as a crude odds ratio [cOR] ≥2.0) between early pregnancy atypical antipsychotic use and conotruncal heart defects (6 exposed cases; cOR: 2.3, 95% confidence interval [CI]: 0.9-6.1), and more specifically Tetralogy of Fallot (3 exposed cases; cOR: 2.5, 95% CI: 0.7-8.8), cleft palate (4 exposed cases, cOR: 2.5, 95% CI: 0.8-7.6), anorectal atresia/stenosis (3 exposed cases, cOR: 2.8, 95% CI: 0.8-9.9), and gastroschisis (3 exposed cases, cOR: 2.1, 95% CI: 0.6-7.3).
Conclusions: Our findings support the close clinical monitoring of pregnant women using atypical antipsychotics. Women treated with atypical antipsychotics generally access healthcare services before pregnancy; efforts to reduce correlates of atypical antipsychotic use might improve maternal and infant health in this population.
Keywords: Antipsychotics; Birth defects; Mental health; Pharmacoepidemiology.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors have no conflicts of interest to report.
Figures

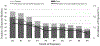
References
-
- American College of Obstetrics and Gynecology Practice Bulletin, 2008. Use of psychiatric medications during pregnancy and lactation. Obstet. Gynecol 111 (4) 1001–1020. - PubMed
-
- Bellet F, Beyens MN, Bernard N, et al. , 2015. Exposure to aripiprazole during embryogenesis: a prospective multicenter cohort study. Pharmacoepidemiol. Drug. Saf 24 (4) 368–380. - PubMed
-
- Cannon M, Jones PB, Murray RM, 2002. Obstetric complications and schizophrenia: historical and meta-analytic review. Am. J. Psychiatry. 159 (7) 1080–1092. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

