Epigenetically upregulated GEFT-derived invasion and metastasis of rhabdomyosarcoma via epithelial mesenchymal transition promoted by the Rac1/Cdc42-PAK signalling pathway
- PMID: 31761617
- PMCID: PMC6921210
- DOI: 10.1016/j.ebiom.2019.10.060
Epigenetically upregulated GEFT-derived invasion and metastasis of rhabdomyosarcoma via epithelial mesenchymal transition promoted by the Rac1/Cdc42-PAK signalling pathway
Erratum in
-
Erratum regarding previously published research papers.EBioMedicine. 2021 Apr;66:103295. doi: 10.1016/j.ebiom.2021.103295. Epub 2021 Apr 9. EBioMedicine. 2021. PMID: 33845237 Free PMC article. No abstract available.
Abstract
Background: Metastasis of rhabdomyosarcoma (RMS) is the primary cause of tumour-related deaths. Previous studies have shown that overexpression of the guanine nucleotide exchange factor T (GEFT) is correlated with a poorer RMS prognosis, but the mechanism remains largely unexplored.
Methods: We focused on determining the influence of the GEFT-Rho-GTPase signalling pathway and the epithelial-mesenchymal transition (EMT) or mesenchymal-epithelial transition (MET) on RMS progression and metastasis by using RMS cell lines, BALB/c nude mice and cells and molecular biology techniques.
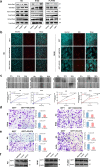
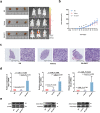
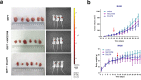
Findings: GEFT promotes RMS cell viability, migration, and invasion; GEFT also inhibits the apoptosis of RMS cells and accelerates the growth and lung metastasis of RMS by activating the Rac1/Cdc42 pathways. Interestingly, GEFT upregulates the expression levels of N-cadherin, Snail, Slug, Twist, Zeb1, and Zeb2 and reduces expression level of E-cadherin. Thus, GEFT influences the expression of markers for EMT and MET in RMS cells via the Rac1/Cdc42-PAK1 pathways. We also found that the level of GEFT gene promoter methylation in RMS is lower than that in normal striated muscle tissue. Significant differences were observed in the level of GEFT gene methylation in different histological subtypes of RMS.
Interpretation: These findings suggest that GEFT accelerates the tumourigenicity and metastasis of RMS by activating Rac1/Cdc42-PAK signalling pathway-induced EMT; thus, it may serve as a novel therapeutic target. FUND: This work was supported by grants from the National Natural Science Foundation of China (81660441, 81460404, and 81160322) and Shihezi University Initiative Research Projects for Senior Fellows (RCZX201447). Funders had no role in the design of the study, data collection, data analysis, interpretation, or the writing of this report.
Keywords: EMT; GEFT; Methylation; Rac1/Cdc42-PAK1 pathways; Rhabdomyosarcoma.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Comment in
-
Epigenetics in rhabdomyosarcoma: cues to new biomarkers and targeted therapies.EBioMedicine. 2020 Feb;52:102673. doi: 10.1016/j.ebiom.2020.102673. Epub 2020 Feb 12. EBioMedicine. 2020. PMID: 32058940 Free PMC article. No abstract available.
References
-
- Doyle L.A. Sarcoma classification: an update based on the 2013 World Health Organization classification of tumors of soft tissue and bone. Cancer. 2014;120(12):1763–1774. - PubMed
-
- Breneman J.C., Lyden E., Pappo A.S., Link M.P., Anderson J.R., Parham D.M. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma – a report from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol. 2003;21(1):78–84. - PubMed
-
- Chaffer C.L., Weinberg R.A. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

