Emergence of SARM1 as a Potential Therapeutic Target for Wallerian-type Diseases
- PMID: 31761689
- PMCID: PMC6980728
- DOI: 10.1016/j.chembiol.2019.11.002
Emergence of SARM1 as a Potential Therapeutic Target for Wallerian-type Diseases
Abstract
Wallerian degeneration is a neuronal death pathway that is triggered in response to injury or disease. Death was thought to occur passively until the discovery of a mouse strain, i.e., Wallerian degeneration slow (WLDS), which was resistant to degeneration. Given that the WLDS mouse encodes a gain-of-function fusion protein, its relevance to human disease was limited. The later discovery that SARM1 (sterile alpha and toll/interleukin receptor [TIR] motif-containing protein 1) promotes Wallerian degeneration suggested the existence of a pathway that might be targeted therapeutically. More recently, SARM1 was found to execute degeneration by hydrolyzing NAD+. Notably, SARM1 knockdown or knockout prevents neuron degeneration in response to a range of insults that lead to peripheral neuropathy, traumatic brain injury, and neurodegenerative disease. Here, we discuss the role of SARM1 in Wallerian degeneration and the opportunities to target this enzyme therapeutically.
Keywords: NAD(+); SARM1; neurodegeneration; therapeutics.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
P.R.T. is a consultant for Disarm Therapeutics.
Figures
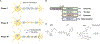


Similar articles
-
SARM1 activation triggers axon degeneration locally via NAD⁺ destruction.Science. 2015 Apr 24;348(6233):453-7. doi: 10.1126/science.1258366. Epub 2015 Apr 23. Science. 2015. PMID: 25908823 Free PMC article.
-
Identification of the first noncompetitive SARM1 inhibitors.Bioorg Med Chem. 2020 Sep 15;28(18):115644. doi: 10.1016/j.bmc.2020.115644. Epub 2020 Jul 17. Bioorg Med Chem. 2020. PMID: 32828421 Free PMC article.
-
Axons Matter: The Promise of Treating Neurodegenerative Disorders by Targeting SARM1-Mediated Axonal Degeneration.Trends Pharmacol Sci. 2020 Apr;41(4):281-293. doi: 10.1016/j.tips.2020.01.006. Epub 2020 Feb 24. Trends Pharmacol Sci. 2020. PMID: 32107050 Review.
-
Sarm1 deletion suppresses TDP-43-linked motor neuron degeneration and cortical spine loss.Acta Neuropathol Commun. 2019 Oct 28;7(1):166. doi: 10.1186/s40478-019-0800-9. Acta Neuropathol Commun. 2019. PMID: 31661035 Free PMC article.
-
SARM1 can be a potential therapeutic target for spinal cord injury.Cell Mol Life Sci. 2022 Feb 28;79(3):161. doi: 10.1007/s00018-022-04195-4. Cell Mol Life Sci. 2022. PMID: 35224705 Free PMC article. Review.
Cited by
-
The Role of NMNAT2/SARM1 in Neuropathy Development.Biology (Basel). 2024 Jan 22;13(1):61. doi: 10.3390/biology13010061. Biology (Basel). 2024. PMID: 38275737 Free PMC article. Review.
-
Base-Exchange Enabling the Visualization of SARM1 Activities in Sciatic Nerve-Injured Mice.ACS Sens. 2023 Feb 24;8(2):767-773. doi: 10.1021/acssensors.2c02317. Epub 2023 Jan 23. ACS Sens. 2023. PMID: 36689294 Free PMC article.
-
SARM1 is a multi-functional NAD(P)ase with prominent base exchange activity, all regulated bymultiple physiologically relevant NAD metabolites.iScience. 2022 Jan 25;25(2):103812. doi: 10.1016/j.isci.2022.103812. eCollection 2022 Feb 18. iScience. 2022. PMID: 35198877 Free PMC article.
-
The SARM1 TIR domain produces glycocyclic ADPR molecules as minor products.PLoS One. 2024 Apr 18;19(4):e0302251. doi: 10.1371/journal.pone.0302251. eCollection 2024. PLoS One. 2024. PMID: 38635746 Free PMC article.
-
Structural basis for RING-Cys-Relay E3 ligase activity and its role in axon integrity.Nat Chem Biol. 2020 Nov;16(11):1227-1236. doi: 10.1038/s41589-020-0598-6. Epub 2020 Aug 3. Nat Chem Biol. 2020. PMID: 32747811 Free PMC article.
References
-
- Adalbert R, Morreale G, Paizs M, Conforti L, Walker SA, Roderick HL, Bootman MD, Siklos L, and Coleman MP (2012). Intra-axonal calcium changes after axotomy in wild-type and slow Wallerian degeneration axons. Neuroscience 225, 44–54. - PubMed
-
- Beirowski B, Babetto E, Coleman MP, and Martin KR (2008). The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur J Neurosci 28, 1166–1179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

