Xenotransplanted Human Cortical Neurons Reveal Species-Specific Development and Functional Integration into Mouse Visual Circuits
- PMID: 31761708
- PMCID: PMC6899440
- DOI: 10.1016/j.neuron.2019.10.002
Xenotransplanted Human Cortical Neurons Reveal Species-Specific Development and Functional Integration into Mouse Visual Circuits
Abstract
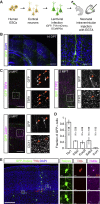
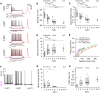
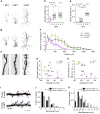
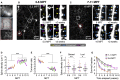
How neural circuits develop in the human brain has remained almost impossible to study at the neuronal level. Here, we investigate human cortical neuron development, plasticity, and function using a mouse/human chimera model in which xenotransplanted human cortical pyramidal neurons integrate as single cells into the mouse cortex. Combined neuronal tracing, electrophysiology, and in vivo structural and functional imaging of the transplanted cells reveal a coordinated developmental roadmap recapitulating key milestones of human cortical neuron development. The human neurons display a prolonged developmental timeline, indicating the neuron-intrinsic retention of juvenile properties as an important component of human brain neoteny. Following maturation, human neurons in the visual cortex display tuned, decorrelated responses to visual stimuli, like mouse neurons, demonstrating their capacity for physiological synaptic integration in host cortical circuits. These findings provide new insights into human neuronal development and open novel avenues for the study of human neuronal function and disease. VIDEO ABSTRACT.
Keywords: brain development; cortical neuron; dendritic spine; gcamp6; human brain evolution; multiphoton imaging; pluripotent stem cell; synapse formation; transplantation; visual cortex.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Astick M., Vanderhaeghen P. From Human Pluripotent Stem Cells to Cortical Circuits. Curr. Top. Dev. Biol. 2018;129:67–98. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

