Apelin+ Endothelial Niche Cells Control Hematopoiesis and Mediate Vascular Regeneration after Myeloablative Injury
- PMID: 31761723
- PMCID: PMC6900750
- DOI: 10.1016/j.stem.2019.10.006
Apelin+ Endothelial Niche Cells Control Hematopoiesis and Mediate Vascular Regeneration after Myeloablative Injury
Abstract
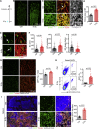
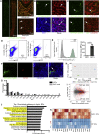
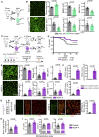
Radiotherapy and chemotherapy disrupt bone vasculature, but the underlying causes and mechanisms enabling vessel regeneration after bone marrow (BM) transplantation remain poorly understood. Here, we show that loss of hematopoietic cells per se, in response to irradiation and other treatments, triggers vessel dilation, permeability, and endothelial cell (EC) proliferation. We further identify a small subpopulation of Apelin-expressing (Apln+) ECs, representing 0.003% of BM cells, that is critical for physiological homeostasis and transplant-induced BM regeneration. Genetic ablation of Apln+ ECs or Apln-CreER-mediated deletion of Kitl and Vegfr2 disrupt hematopoietic stem cell (HSC) maintenance and contributions to regeneration. Consistently, the fraction of Apln+ ECs increases substantially after irradiation and promotes normalization of the bone vasculature in response to VEGF-A, which is provided by transplanted hematopoietic stem and progenitor cells (HSPCs). Together, these findings reveal critical functional roles for HSPCs in maintaining vascular integrity and for Apln+ ECs in hematopoiesis, suggesting potential targets for improving BM transplantation.
Keywords: Apln (Apelin); Esm1; VEGF; VEGFR; bone marrow transplantation; endothelial cell heterogeneity; hematopoietic stem cell; irradiation; stem cell niche; vessel regeneration.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures





Comment in
-
Mutualism in the Marrow.Cell Stem Cell. 2019 Dec 5;25(6):731-733. doi: 10.1016/j.stem.2019.11.007. Cell Stem Cell. 2019. PMID: 31809734
Similar articles
-
Piezo1-mediated mechanosensation in bone marrow macrophages promotes vascular niche regeneration after irradiation injury.Theranostics. 2022 Jan 16;12(4):1621-1638. doi: 10.7150/thno.64963. eCollection 2022. Theranostics. 2022. PMID: 35198061 Free PMC article.
-
VEGF-C protects the integrity of the bone marrow perivascular niche in mice.Blood. 2020 Oct 15;136(16):1871-1883. doi: 10.1182/blood.2020005699. Blood. 2020. PMID: 32842144 Free PMC article.
-
Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration.Blood. 2012 Jun 7;119(23):5429-37. doi: 10.1182/blood-2011-11-393645. Epub 2012 May 2. Blood. 2012. PMID: 22553313 Free PMC article.
-
Complexity of bone marrow hematopoietic stem cell niche.Int J Hematol. 2017 Jul;106(1):45-54. doi: 10.1007/s12185-017-2262-9. Epub 2017 May 22. Int J Hematol. 2017. PMID: 28534115 Free PMC article. Review.
-
Regulation of hematopoietic stem cells in the niche.Sci China Life Sci. 2015 Dec;58(12):1209-15. doi: 10.1007/s11427-015-4960-y. Epub 2015 Nov 13. Sci China Life Sci. 2015. PMID: 26563156 Review.
Cited by
-
Role of ex vivo Expanded Mesenchymal Stromal Cells in Determining Hematopoietic Stem Cell Transplantation Outcome.Front Cell Dev Biol. 2021 May 4;9:663316. doi: 10.3389/fcell.2021.663316. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34017834 Free PMC article. Review.
-
Prior COVID-19 infection may increase risk for developing endothelial dysfunction following hematopoietic cell transplantation.Front Oncol. 2023 Jan 17;12:1000215. doi: 10.3389/fonc.2022.1000215. eCollection 2022. Front Oncol. 2023. PMID: 36733348 Free PMC article.
-
Organ-Specific Endothelial Dysfunction Following Total Body Irradiation Exposure.Toxics. 2022 Dec 1;10(12):747. doi: 10.3390/toxics10120747. Toxics. 2022. PMID: 36548580 Free PMC article. Review.
-
Vascular adhesion protein-1 defines a unique subpopulation of human hematopoietic stem cells and regulates their proliferation.Cell Mol Life Sci. 2021 Dec;78(23):7851-7872. doi: 10.1007/s00018-021-03977-6. Epub 2021 Nov 1. Cell Mol Life Sci. 2021. PMID: 34719737 Free PMC article.
-
Modulation of APLNR Signaling Is Required during the Development and Maintenance of the Hematopoietic System.Stem Cell Reports. 2021 Apr 13;16(4):727-740. doi: 10.1016/j.stemcr.2021.02.003. Epub 2021 Mar 4. Stem Cell Reports. 2021. PMID: 33667414 Free PMC article.
References
-
- Aguirre A., Blázquez-Prieto J., Amado-Rodriguez L., López-Alonso I., Batalla-Solís E., González-López A., Sánchez-Pérez M., Mayoral-Garcia C., Gutiérrez-Fernández A., Albaiceta G.M. Matrix metalloproteinase-14 triggers an anti-inflammatory proteolytic cascade in endotoxemia. J. Mol. Med. (Berl.) 2017;95:487–497. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

