Efficacy of alemtuzumab in relapsing-remitting MS patients who received additional courses after the initial two courses: Pooled analysis of the CARE-MS, extension, and TOPAZ studies
- PMID: 31762387
- PMCID: PMC7720359
- DOI: 10.1177/1352458519888610
Efficacy of alemtuzumab in relapsing-remitting MS patients who received additional courses after the initial two courses: Pooled analysis of the CARE-MS, extension, and TOPAZ studies
Abstract
Background: Alemtuzumab is given as two annual courses. Patients with continued disease activity may receive as-needed additional courses.
Objective: To evaluate efficacy and safety of additional alemtuzumab courses in the CARE-MS (Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis) studies and their extensions.
Methods: Subgroups were based on the number of additional alemtuzumab courses received. Exclusion criteria: other disease-modifying therapy (DMT); <12-month follow-up after last alemtuzumab course.
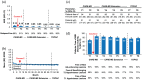
Results: In the additional-courses groups, Courses 3 and 4 reduced annualized relapse rate (12 months before: 0.73 and 0.74, respectively; 12 months after: 0.07 and 0.08). For 36 months after Courses 3 and 4, 89% and 92% of patients were free of 6-month confirmed disability worsening, respectively, with 20% and 26% achieving 6-month confirmed disability improvement. Freedom from magnetic resonance imaging (MRI) disease activity increased after Courses 3 and 4 (12 months before: 43% and 53%, respectively; 12 months after: 73% and 74%). Safety was similar across groups; serious events occurred irrespective of the number of courses.
Conclusion: Additional alemtuzumab courses significantly improved outcomes, without increased safety risks, in CARE-MS patients with continued disease activity after Course 2. How this compares to outcomes if treatment is switched to another DMT instead remains unknown.
Trial registration: ClinicalTrials.gov NCT00530348 NCT00548405 NCT00930553 NCT02255656.
Keywords: Alemtuzumab; efficacy; multiple sclerosis; relapse; retreatment; safety.
Conflict of interest statement
Figures



References
-
- Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet 2012; 380(9856): 1819–1828. - PubMed
-
- Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012; 380(9856): 1829–1839. - PubMed

