Humanized mice for immune checkpoint blockade in human solid tumors
- PMID: 31763362
- PMCID: PMC6872471
Humanized mice for immune checkpoint blockade in human solid tumors
Abstract
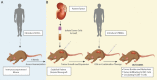
Immunotherapy, specifically research involving immune checkpoint blockers (ICBs), has become a popular trend in anticancer research over the last three years. Due to the difficulties and often poor translation of results from in-vitro models, in-vivo models have become more relevant than ever. With the discovery of NOD, Prkdcscid , and Il2rγ-/- mutations, patient-derived xenograft (PDX) mouse models were developed, providing an ideal environment for ICBs testing. By implanting a PDX with either CD34+ or peripheral blood mononuclear cells, we can create a human immune system capable of mounting a response against tumor burden. These animal models are currently being used to study molecular mechanisms, test drug efficacy, and trial drug combinations. Others have found use for these humanized mouse models as surrogates to represent otherwise uncommon diseases. Limitations remain with regards to what the models are capable of, but in the short amount of time between the development of these models and heightened interest in ICBs, these mice have already shown utility for future developments in the field of immunotherapy.
Keywords: Humanized mice; immune checkpoint blockers; immunotherapy; patient derived xenografts.
AJCEU Copyright © 2019.
Conflict of interest statement
None.
Figures
Similar articles
-
Characterization of immune responses to anti-PD-1 mono and combination immunotherapy in hematopoietic humanized mice implanted with tumor xenografts.J Immunother Cancer. 2019 Feb 8;7(1):37. doi: 10.1186/s40425-019-0518-z. J Immunother Cancer. 2019. PMID: 30736857 Free PMC article.
-
Testing Cancer Immunotherapy in a Human Immune System Mouse Model: Correlating Treatment Responses to Human Chimerism, Therapeutic Variables and Immune Cell Phenotypes.Front Immunol. 2021 Mar 29;12:607282. doi: 10.3389/fimmu.2021.607282. eCollection 2021. Front Immunol. 2021. PMID: 33854497 Free PMC article.
-
Establishment of Humanized Mice from Peripheral Blood Mononuclear Cells or Cord Blood CD34+ Hematopoietic Stem Cells for Immune-Oncology Studies Evaluating New Therapeutic Agents.Curr Protoc Pharmacol. 2020 Jun;89(1):e77. doi: 10.1002/cpph.77. Curr Protoc Pharmacol. 2020. PMID: 32453514
-
Humanized SCID mouse models for biomedical research.Curr Top Microbiol Immunol. 2008;324:25-51. doi: 10.1007/978-3-540-75647-7_2. Curr Top Microbiol Immunol. 2008. PMID: 18481451 Review.
-
The development and improvement of immunodeficient mice and humanized immune system mouse models.Front Immunol. 2022 Oct 19;13:1007579. doi: 10.3389/fimmu.2022.1007579. eCollection 2022. Front Immunol. 2022. PMID: 36341323 Free PMC article. Review.
Cited by
-
Golden Syrian Hamster Models for Cancer Research.Cells. 2022 Aug 3;11(15):2395. doi: 10.3390/cells11152395. Cells. 2022. PMID: 35954238 Free PMC article. Review.
-
Mass spectrometry-based tear proteomics for noninvasive biomarker discovery.Mass Spectrom Rev. 2022 Sep;41(5):842-860. doi: 10.1002/mas.21691. Epub 2021 Mar 24. Mass Spectrom Rev. 2022. PMID: 33759206 Free PMC article. Review.
-
Animal model considerations for chordoma research: reproducing the tumor microenvironment in vivo with humanized mice.Front Oncol. 2024 Mar 13;14:1330254. doi: 10.3389/fonc.2024.1330254. eCollection 2024. Front Oncol. 2024. PMID: 38544830 Free PMC article. Review.
-
Immunity, immunotherapy, and rectal cancer: A clinical and translational science review.Transl Res. 2021 May;231:124-138. doi: 10.1016/j.trsl.2020.12.002. Epub 2020 Dec 8. Transl Res. 2021. PMID: 33307273 Free PMC article. Review.
-
From Cancer to Immune-Mediated Diseases and Tolerance Induction: Lessons Learned From Immune Oncology and Classical Anti-cancer Treatment.Front Immunol. 2020 Jul 8;11:1423. doi: 10.3389/fimmu.2020.01423. eCollection 2020. Front Immunol. 2020. PMID: 32733473 Free PMC article. Review.
References
-
- Kochanek KD, Murphy SL, Xu J, Arias E. Deaths: final data for 2017. Natl Vital Stat Rep. 2019;68:77. - PubMed
-
- Kozlowska AK, Kaur K, Topchyan P, Jewett A. Novel strategies to target cancer stem cells by NK cells; studies in humanized mice. Front Biosci (Landmark Ed) 2017;22:370–384. - PubMed
-
- Pyo KH, Kim JH, Lee JM, Kim SE, Cho JS, Lim SM, Cho BC. Promising preclinical platform for evaluation of immuno-oncology drugs using Hu-PBL-NSG lung cancer models. Lung Cancer. 2019;127:112–121. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
