Evaluation of metal-organic framework NH2-MIL-101(Fe) as an efficient sorbent for dispersive micro-solid phase extraction of phenolic pollutants in environmental water samples
- PMID: 31763487
- PMCID: PMC6861588
- DOI: 10.1016/j.heliyon.2019.e02848
Evaluation of metal-organic framework NH2-MIL-101(Fe) as an efficient sorbent for dispersive micro-solid phase extraction of phenolic pollutants in environmental water samples
Abstract
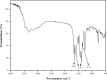
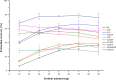
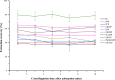
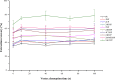
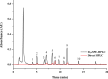
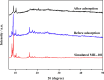
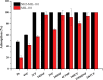
This work proposes an application of amine-functionalized metal-organic framework (NH2-MIL-101(Fe)) as sorbent for dispersive micro-solid phase extraction (D-μSPE) of ten priority phenolic pollutants. The sorbent was simply synthesized under facile condition. The entire D-μSPE process was optimized by studying the effect of experimental parameters affecting the extraction recovery of the target analytes. The final extract was analyzed using high performance liquid chromatography with photodiode array detector. Under the optimum condition, the proposed procedure can be applied for wide linear calibration ranges between 1.25-5000 μg L-1 with the correlation coefficients of greater than 0.9900. The limits of detection (LODs) and limits of quantitation (LOQs) were in the ranges of 0.4-9.5 μg L-1 and 1.25-30 μg L-1, respectively. The precision evaluated in terms of the relative standard deviations (RSDs) of the intra- and inter-day determinations of the phenol compounds at their LOQ concentrations were below 13.9% and 12.2%, respectively. High enrichment factors up to 120 were reached. The developed method has been successfully applied to determine phenol residues in environmental water samples. The satisfactory recoveries obtained by spiking phenol standards at two different concentrations (near LOQs and 5 times as high as LOQs) ranged from 68.4-114.4%. The results demonstrate that the NH2-MIL-101(Fe) material is promising sorbent in the D-μSPE of phenolic pollutants.
Keywords: Analytical chemistry; Dispersive solid-phase extraction; Environmental science; HPLC; Metal-organic framework; Phenol.
© 2019 The Author(s).
Figures







Similar articles
-
A Novel Method for Monitoring N-Nitrosamines Impurities Using NH2-MIL-101(Fe) Mediated Dispersive Micro-Solid Phase Extraction Coupled with LC-MS/MS in Biopharmaceuticals.J Pharm Sci. 2023 Nov;112(11):2783-2789. doi: 10.1016/j.xphs.2023.07.017. Epub 2023 Jul 20. J Pharm Sci. 2023. PMID: 37481163
-
Simple magnetization of Fe3 O4 /MIL-53(Al)-NH2 for a rapid vortex-assisted dispersive magnetic solid-phase extraction of phenol residues in water samples.J Sep Sci. 2020 Aug;43(15):3083-3092. doi: 10.1002/jssc.202000426. Epub 2020 Jun 14. J Sep Sci. 2020. PMID: 32445251
-
Solvent-loaded metal-organic framework of type MIL-101(Cr)-NH2 for the dispersive solid-phase extraction and UHPLC-MS/MS analysis of herbicides from paddy field waters.Mikrochim Acta. 2021 Jan 7;188(2):30. doi: 10.1007/s00604-020-04661-5. Mikrochim Acta. 2021. PMID: 33415463
-
A nanosized magnetic metal-organic framework of type MIL-53(Fe) as an efficient sorbent for coextraction of phenols and anilines prior to their quantitation by HPLC.Mikrochim Acta. 2019 Aug 2;186(9):597. doi: 10.1007/s00604-019-3698-9. Mikrochim Acta. 2019. PMID: 31375929
-
Advancements in effervescent-assisted dispersive micro-solid phase extraction for the analysis of emerging pollutants.Anal Chim Acta. 2024 Oct 9;1325:342891. doi: 10.1016/j.aca.2024.342891. Epub 2024 Jun 19. Anal Chim Acta. 2024. PMID: 39244296 Review.
Cited by
-
Porous NH2-MIL-101(Fe) metal organic framework for effective photocatalytic degradation of azo dye in wastewater treatment.Heliyon. 2022 Jul 12;8(7):e09942. doi: 10.1016/j.heliyon.2022.e09942. eCollection 2022 Jul. Heliyon. 2022. PMID: 35865975 Free PMC article.
-
MIL-101 (Fe)-NH2 metal-organic framework/graphene oxide nanocomposite modified screen-printed carbon electrode for electrochemical sensing of 2,4-dichlorophenol in water samples.Heliyon. 2025 Jan 25;11(3):e42285. doi: 10.1016/j.heliyon.2025.e42285. eCollection 2025 Feb 15. Heliyon. 2025. PMID: 39968152 Free PMC article.
-
Polydopamine-Modified Metal-Organic Frameworks, NH2-Fe-MIL-101, as pH-Sensitive Nanocarriers for Controlled Pesticide Release.Nanomaterials (Basel). 2020 Oct 10;10(10):2000. doi: 10.3390/nano10102000. Nanomaterials (Basel). 2020. PMID: 33050439 Free PMC article.
-
FeNP@MIL-101(Fe)-Based Carbon Nanotube Composite for Energy Storage Applications.ACS Omega. 2024 May 28;9(23):24546-24557. doi: 10.1021/acsomega.4c00590. eCollection 2024 Jun 11. ACS Omega. 2024. PMID: 38882151 Free PMC article.
References
-
- Serra-Crespo P., Berger R., Yang W., Gascon J., Kapteijn F. Separation of CO2/CH4 mixtures over NH2-MIL-53-An experimental and modelling study. Chem. Eng. Sci. 2015;124:96–108.
-
- Boutin A., Couck S., Coudert F., Serra-Crespo P., Gascon J., Kapteijn F., Fushs A.H., Denayer J.F.M. Thermodynamic analysis of the breathing of amino-functionalized MIL-53(Al) upon CO2 adsorption. Microporous Mesoporous Mater. 2011;140:108–113.
-
- Serra-Crespo P., Ramos-Fernandez E.V., Gascon J., Kapteijn F. Synthesis and characterization of an amino functionalized MIL-101(Al): separation and catalytic properties. Chem. Mater. 2011;23:2565–2572.
-
- Gascon J., Aktay U., Hernandez-Alonso M.D., van Klink G.P.M., Kapteijn F. Amino-based metal-organic frameworks as stable, highly active basic catalysts. J. Catal. 2009;261:75–87.
-
- Hu Z., Deibert B.J., Li J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014;43:5815–5840. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

