Anti-CD34-Grafted Magnetic Nanoparticles Promote Endothelial Progenitor Cell Adhesion on an Iron Stent for Rapid Endothelialization
- PMID: 31763571
- PMCID: PMC6868894
- DOI: 10.1021/acsomega.9b03016
Anti-CD34-Grafted Magnetic Nanoparticles Promote Endothelial Progenitor Cell Adhesion on an Iron Stent for Rapid Endothelialization
Abstract
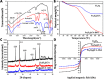
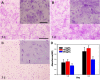
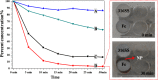
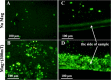
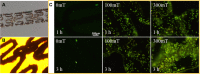
Iron stents, with superior mechanical properties and controllable degradation behavior, have potential for use as feasible substitutes for nondegradable stents in the treatment of coronary artery occlusion. However, corrosion renders the iron surface hard to modify with biological molecules to accelerate endothelialization and solve restenosis. The objective of this study is to demonstrate the feasibility of using endothelial progenitor cells (EPCs) to rapidly adhere onto iron surfaces with the assistance of anti-CD34-modified magnetic nanoparticles. Transmission electron microscopy, Fourier transform infrared spectroscopy, Thermogravimetric analysis, XRD, and anti-CD34 immunofluorescence suggested that anti-CD34 and citric acid were successfully modified onto Fe3O4, and Prussian blue staining demonstrated the selectivity of the as-prepared nanoparticles for EPCs. Under an external magnetic field (EMF), numerous nanoparticles or EPCs attached onto the surface of iron pieces, particularly the side of the iron pieces exposed to flow conditions, because iron could be magnetized under the EMF, and the magnetized iron has an edge effect. However, the uniform adhesion of EPCs on the iron stent was completed because of the weakening edge effect, and the sum of adherent EPCs was closely linked with the magnetic field (MF) intensity, which was validated by the complete covering of EPCs on the iron stent upon exposure to a 300 mT EMF within 3 h, whereas almost no cells were observed on the iron stent without an EMF. These results verify that this method can efficiently promote EPC capture and endothelialization of iron stents.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Zaman A.; de Winter R. J.; Kogame N.; Chang C. C.; Modolo R.; Spitzer E.; Tonino P.; Hofma S.; Zurakowski A.; Smits P. C.; Prokopczuk J.; Moreno R.; Choudhury A.; Petrov I.; Cequier A.; Kukreja N.; Hoye A.; Iniguez A.; Ungi I.; Serra A.; Gil R. J.; Walsh S.; Tonev G.; Mathur A.; Merkely B.; Colombo A.; Ijsselmuiden S.; Soliman O.; Kaul U.; Onuma Y.; Serruys P. W. Safety and efficacy of a sirolimus-eluting coronary stent with ultra-thin strut for treatment of atherosclerotic lesions (TALENT): a prospective multicentre randomised controlled trial. Lancet 2019, 393, 987–997. 10.1016/s0140-6736(18)32467-x. - DOI - PubMed
-
- Boccafoschi F.; Fusaro L.; Cannas M.. 15-Immobilization of peptides on cardiovascular stent. In Functionalised Cardiovascular Stents; Wall J. G., Podbielska H., Wawrzyńska M., Eds.; Woodhead Publishing, 2018, pp 305–318.
-
- McMahon C. J.; El-Said H. G.; Grifka R. G.; Fraley J. K.; Nihill M. R.; Mullins C. E. Redilation of endovascular stents in congenital heart disease: factors implicated in the development of restenosis and neointimal proliferation. J. Am. Coll. Cardiol. 2001, 38, 521–526. 10.1016/s0735-1097(01)01406-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
