Immunophenotypic Detection of Measurable Residual (Stem Cell) Disease Using LAIP Approach in Acute Myeloid Leukemia
- PMID: 31763792
- PMCID: PMC6856793
- DOI: 10.1002/cpcy.66
Immunophenotypic Detection of Measurable Residual (Stem Cell) Disease Using LAIP Approach in Acute Myeloid Leukemia
Abstract
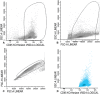
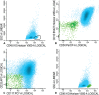
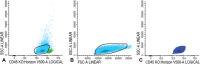
Half of the patients with acute myeloid leukemia (AML), who achieve complete remission after chemotherapy treatment, will ultimately experience a relapse. Measurable residual disease (MRD) is an important post-treatment risk factor in AML, because it gives additional information about the depth of the remission. Within MRD, the small population of leukemic stem cells (LSCs) is thought to be at the base of the actual relapse. In this protocol, the flow cytometric detection of MRD and LSCs herein is outlined. We give a detailed overview of the sampling procedures for optimal multiparameter flow cytometry assessment of both MRD and LSC, using leukemia associated immunophenotypes (LAIPs) and LSC markers. Moreover, an overview of the gating strategies to detect LAIPs and LSC markers is provided. This protocol serves as guidance for flow cytometric detection of measurable residual (stem cell) disease necessary for proper therapeutic decision making in AML patients. © 2019 The Authors. Basic Protocol 1: Immunophenotypic LAIP detection for measurable residual disease monitoring Basic Protocol 2: Immunophenotypic detection of CD34+CD38- leukemic stem cells.
Keywords: acute myeloid leukemia (AML); leukemia associated immunophenotypes (LAIPs); leukemic stem cells (LSCs); measurable residual disease (MRD); multiparameter flow cytometry (MFC).
© 2019 The Authors.
Figures






References
-
- Blair, B. A. , Hogge, D. E. , & Sutherland, H. J. (1998). Most acute myeloid leukemia progenitor cells with long‐term proliferative ability in vitro and in vivo have the phenotype CD34+/CD71‐/HLA‐DR‐. Blood, 92(11), 4325–4335. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

