HIV-1 genetic diversity to estimate time of infection and infer adherence to preexposure prophylaxis
- PMID: 31764095
- PMCID: PMC11000142
- DOI: 10.1097/QAD.0000000000002390
HIV-1 genetic diversity to estimate time of infection and infer adherence to preexposure prophylaxis
Abstract
Objective: To estimate time of HIV infection in participants from the Bangkok Tenofovir Study (BTS) with daily oral tenofovir disoproxil fumarate (TDF) for preexposure prophylaxis (PrEP) and relate infection with adherence patterns.
Design: We used the diversity structure of the virus population at the first HIV RNA-positive sample to estimate the date of infection, and mapped these estimates to medication diaries obtained under daily directly observed therapy (DOT).
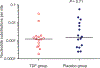
Methods: HIV genetic diversity was investigated in all 17 PrEP breakthrough infections and in 16 placebo recipients. We generated 10-25 HIV env sequences from each participant by single genome amplification, and calculated time since infection (and 95% confidence interval) using Poisson models of early virus evolution. Study medication diaries obtained under daily DOT were then used to compute the number of missed TDF doses at the approximate date of infection.
Results: Fifteen of the 17 PrEP breakthrough infections were successfully amplified. Of these, 13 were initiated by a single genetic variant and generated reliable estimates of time since infection (median = 47 [IQR = 35] days). Eleven of these 13 were under daily DOT at the estimated time of infection. Analysis of medication diaries in these 11 participants showed 100% adherence in five, 90-95% adherence in two, 55% adherence in one, and nonadherence in three.
Conclusion: We estimated time of infection in participants from BTS and found several infections when high levels of adherence to TDF were reported. Our results suggest that the biological efficacy of daily TDF against parenteral HIV exposure is not 100%.
Conflict of interest statement
Conflicts of interest
J.G.G.-L. and W.H. are named on US Government patents on ‘Inhibition of HIV infection through chemoprophylaxis’, and on patent applications on ‘HIV post-exposure prophylaxis’ and ‘HIV pre-exposure prophylaxis’.
Figures


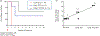
Comment in
-
Narrowing down the causes of failure of tenofovir (only) for preexposure prophylaxis.AIDS. 2019 Dec 1;33(15):2437-2438. doi: 10.1097/QAD.0000000000002391. AIDS. 2019. PMID: 31764109 No abstract available.
References
-
- HIV/AIDS fact sheet World Health organization [updated 2017]. Available at: http://www.who.int/mediacentre/factsheets/fs360/en/. [Accessed 10 July 2019]
-
- Riddell J, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA 2018; 319:1261–1268. - PubMed
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. , TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

