RANIBIZUMAB TREATMENT IN TREATMENT-NAIVE NEOVASCULAR AGE-RELATED MACULAR DEGENERATION: Results From LUMINOUS, a Global Real-World Study
- PMID: 31764612
- PMCID: PMC7447127
- DOI: 10.1097/IAE.0000000000002670
RANIBIZUMAB TREATMENT IN TREATMENT-NAIVE NEOVASCULAR AGE-RELATED MACULAR DEGENERATION: Results From LUMINOUS, a Global Real-World Study
Abstract
Purpose: To evaluate the effectiveness, safety, and treatment patterns of ranibizumab 0.5 mg in treatment-naive patients with neovascular age-related macular degeneration enrolled in LUMINOUS study.
Methods: This 5-year, prospective, multicenter, observational study recruited 30,138 adult patients (treatment-naive or previously treated with ranibizumab or other ocular treatments) who were treated according to the local ranibizumab label.
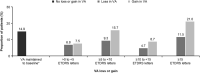
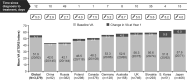
Results: Six thousand two hundred and forty-one treatment-naive neovascular age-related macular degeneration patients were recruited. Baseline (BL) demographics were, mean (SD) age 75.0 (10.2) years, 54.9% females, and 66.5% Caucasian. The mean (SD) visual acuity (VA; letters) gain at 1 year was 3.1 (16.51) (n = 3,379; BLVA, 51.9 letters [Snellen: 20/92]) with a mean (SD) of 5.0 (2.7) injections and 8.8 (3.3) monitoring visits. Presented by injection frequencies <3 (n = 537), 3 to 6 (n = 1,924), and >6 (n = 918), visual acuity gains were 1.6 (14.93), 3.3 (16.57), and 3.7 (17.21) letters, respectively. Stratified by BLVA <23 (n = 382), 23 to <39 (n = 559), 39 to <60 (n = 929), 60 to <74 (n = 994), and ≥74 (n = 515), visual acuity change was 12.6 (20.63), 6.7 (17.88), 3.6 (16.41), 0.3 (13.83), and -3.0 (11.82) letters, respectively. The incidence of ocular/nonocular adverse events was 8.2%/12.8% and serious adverse events were 0.9%/7.4%, respectively.
Conclusion: These results demonstrate the effectiveness and safety of ranibizumab in treatment-naive neovascular age-related macular degeneration patients.
Conflict of interest statement
F. G. Holz: Consultant—Acucela, Bayer, Boehringer-Ingelheim, Genentech, Heidelberg Engineering, LIN Bioscience, Novartis, Pixium, Roche, and Zeiss; Grants—Allergan, Carl Zeiss Meditec, Genentech, Heidelberg Engineering, Nightstar, Novartis, Optos, Pixium, and Roche; Lecture fees—Bayer, Genentech, Heidelberg Engineering, Novartis, Roche, and Zeiss. M. S. Figueroa: Consultant—Alcon, Allergan, Bayer, Novartis, Roche, and Zeiss. F. Bandello: Consultant—Allergan, Bayer, Boehringer-Ingelheim, Hofmann La Roche, Novartis, NTC Pharma, Sifi, Sooft, Thrombogenics, and Zeiss; Board membership, expert testimony, and lecture fees—Allergan, Bayer, Boehringer-Ingelheim, Hofmann La Roche, Novartis, NTC Pharma, Sifi, Sooft, Thrombogenics, and Zeiss; Y. Yang: Research grants—Alcon, Allergan, Alimera Sciences, Bayer, Thrombogenics; Consultant—Allergan, Alimera Sciences, Bayer, Pfizer; Honoraria from Alcon, Allergan, Alimera Sciences, Bayer, Novartis, Pfizer, and Thrombogenics; Advisory board—Novartis. M. Ohji: Grant—Alcon, Hoya Corp, Kowa Pharmaceutical, Novartis, Otsuka Pharmaceutical, Pfizer, Santen Pharmaceutical, Senju Pharmaceutical, and Topcon; Personal fees—Alcon, Allergan, Bayer, Bausch & Lomb, Carl Zeiss, Chugai Pharmaceutical, Chuo Sangio, Hoya Corp, Kowa Pharmaceutical, MSD, Novartis, Otsuka Pharmaceutical, Pfizer, RE Medical Inc, Santen Pharmaceutical, Senju Pharmaceutical, Sanwa Kagaku Kenkyusho, and Topcon. H. Dai: Research grants and nonfinancial support—Novartis. S. Sharma: Financial support—Alcon, Allergan, Bausch and Lomb, Bayer, Genentech, Health Canada, Novartis, Roche, and the Canadian Institutes of Health Research; Consultant—Alcon, Allergan, Bausch and Lomb, Bayer, Genentech, Health Canada, Novartis, Roche, and the Canadian Institutes of Health Research. C. Dunger-Baldauf: Employee—Novartis Pharma AG, Basel, Switzerland. S. Lacey: Employee—Novartis Pharmaceuticals UK Limited, Surrey, Frimley, Camberley, United Kingdom. W. Macfadden: Employee—Novartis Pharma AG, Basel, Switzerland. P. Mitchell: Consultant—Abbott, Allergan, Bayer, Genentech, Novartis, and Roche; Financial support—Abbott, Allergan, Bayer, Genentech, Novartis, and Roche. The remaining author has no conflict of interests to disclose.
Figures


Comment in
-
Correspondence.Retina. 2020 Sep;40(9):e49-e51. doi: 10.1097/IAE.0000000000002908. Retina. 2020. PMID: 32842091 No abstract available.
-
Reply.Retina. 2020 Sep;40(9):e51-e53. doi: 10.1097/IAE.0000000000002909. Retina. 2020. PMID: 32842092 No abstract available.
References
-
- Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA 2004;291:1900–1901. - PubMed
-
- Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–572. - PubMed
-
- Kawasaki R, Yasuda M, Song SJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology 2010;117:921–927. - PubMed
-
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–e116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

