Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first-line treatment in adults with transplant-ineligible multiple myeloma: a network meta-analysis
- PMID: 31765002
- PMCID: PMC6876545
- DOI: 10.1002/14651858.CD013487
Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first-line treatment in adults with transplant-ineligible multiple myeloma: a network meta-analysis
Abstract
Background: Multiple myeloma is a bone marrow-based hematological malignancy accounting for approximately two per cent of cancers. First-line treatment for transplant-ineligible individuals consists of multiple drug combinations of bortezomib (V), lenalidomide (R), or thalidomide (T). However, access to these medicines is restricted in many countries worldwide.
Objectives: To assess and compare the effectiveness and safety of multiple drug combinations of V, R, and T for adults with newly diagnosed transplant-ineligible multiple myeloma and to inform an application for the inclusion of these medicines into the World Health Organization's (WHO) list of essential medicines.
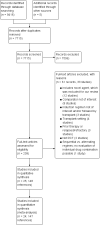
Search methods: We searched CENTRAL and MEDLINE, conference proceedings and study registries on 14 February 2019 for randomised controlled trials (RCTs) comparing multiple drug combinations of V, R and T for adults with newly diagnosed transplant-ineligible multiple myeloma.
Selection criteria: We included RCTs comparing combination therapies of V, R, and T, plus melphalan and prednisone (MP) or dexamethasone (D) for first-line treatment of adults with transplant-ineligible multiple myeloma. We excluded trials including adults with relapsed or refractory disease, trials comparing drug therapies to other types of therapy and trials including second-generation novel agents.
Data collection and analysis: Two review authors independently extracted data and assessed risk of bias of included trials. As effect measures we used hazard ratios (HRs) for overall survival (OS) and progression-free survival (PFS) and risk ratios (RRs) for adverse events. An HR or RR < 1 indicates an advantage for the intervention compared to the main comparator MP. Where available, we extracted quality of life (QoL) data (scores of standardised questionnaires). Results quoted are from network meta-analysis (NMA) unless stated.
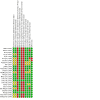
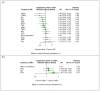
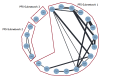
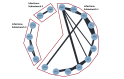
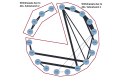
Main results: We included 25 studies (148 references) comprising 11,403 participants and 21 treatment regimens. Treatments were differentiated between restricted treatment duration (treatment with a pre-specified amount of cycles) and continuous therapy (treatment administered until disease progression, the person becomes intolerant to the drug, or treatment given for a prolonged period). Continuous therapies are indicated with a "c". Risk of bias was generally high across studies due to the open-label study design. Overall survival (OS) Evidence suggests that treatment with RD (HR 0.63 (95% confidence interval (CI) 0.40 to 0.99), median OS 55.2 months (35.2 to 87.0)); TMP (HR 0.75 (95% CI 0.58 to 0.97), median OS: 46.4 months (35.9 to 60.0)); and VRDc (HR 0.49 (95% CI 0.26 to 0.92), median OS 71.0 months (37.8 to 133.8)) probably increases survival compared to median reported OS of 34.8 months with MP (moderate certainty). Treatment with VMP may result in a large increase in OS, compared to MP (HR 0.70 (95% CI 0.45 to 1.07), median OS 49.7 months (32.5 to 77.3)), low certainty). Progression-free survival (PFS) Treatment withRD (HR 0.65 (95% CI0.44 to 0.96), median PFS: 24.9 months (16.9 to 36.8)); TMP (HR 0.63 (95% CI 0.50 to 0.78), median PFS:25.7 months (20.8 to 32.4)); VMP (HR 0.56 (95% CI 0.35 to 0.90), median PFS: 28.9 months (18.0 to 46.3)); and VRDc (HR 0.34 (95% CI 0.20 to 0.58), median PFS: 47.6 months (27.9 to 81.0)) may result in a large increase in PFS (low certainty) compared to MP (median reported PFS: 16.2 months). Adverse events The risk of polyneuropathies may be lower with RD compared to treatment with MP (RR 0.57 (95% CI 0.16 to 1.99), risk for RD: 0.5% (0.1 to 1.8), mean reported risk for MP: 0.9% (10 of 1074 patients affected), low certainty). However, the CIs are also compatible with no difference or an increase in neuropathies. Treatment with TMP (RR 4.44 (95% CI1.77 to 11.11), risk: 4.0% (1.6 to 10.0)) and VMP (RR 88.22 (95% CI 5.36 to 1451.11), risk: 79.4% (4.8 to 1306.0)) probably results in a large increase in polyneuropathies compared to MP (moderate certainty). No study reported the amount of participants with grade ≥ 3 polyneuropathies for treatment with VRDc. VMP probably increases the proportion of participants with serious adverse events (SAEs) compared to MP (RR 1.28 (95% CI 1.06 to 1.54), risk for VMP: 46.2% (38.3 to 55.6), mean risk for MP: 36.1% (177 of 490 patients affected), moderate certainty). RD, TMP, and VRDc were not connected to MP in the network and the risk of SAEs could not be compared. Treatment with RD (RR 4.18 (95% CI 2.13 to 8.20), NMA-risk: 38.5% (19.6 to 75.4)); and TMP (RR 4.10 (95% CI 2.40 to 7.01), risk: 37.7% (22.1 to 64.5)) results in a large increase of withdrawals from the trial due to adverse events (high certainty) compared to MP (mean reported risk: 9.2% (77 of 837 patients withdrew)). The risk is probably slightly increased with VMP (RR 1.06 (95% CI 0.63 to 1.81), risk: 9.75% (5.8 to 16.7), moderate certainty), while it is much increased with VRDc (RR 8.92 (95% CI 3.82 to 20.84), risk: 82.1% (35.1 to 191.7), high certainty) compared to MP. Quality of life QoL was reported in four studies for seven different treatment regimens (MP, MPc, RD, RMP, RMPc, TMP, TMPc) and was measured with four different tools. Assessment and reporting differed between studies and could not be meta-analysed. However, all studies reported an improvement of QoL after initiation of anti-myeloma treatment for all assessed treatment regimens.
Authors' conclusions: Based on our four pre-selected comparisons of interest, continuous treatment with VRD had the largest survival benefit compared with MP, while RD and TMP also probably considerably increase survival. However, treatment combinations of V, R, and T also substantially increase the incidence of AEs, and lead to a higher risk of treatment discontinuation. Their effectiveness and safety profiles may best be analysed in further randomised head-to-head trials. Further trials should focus on consistent reporting of safety outcomes and should use a standardised instrument to evaluate QoL to ensure comparability of treatment-combinations.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
NS: none known.
VP: none known.
PL: none known.
TJ: none known.
IM: none known.
CS: The author has received honoraria and travel support from Janssen, Celgene, Novartis, Bristol Myers Squibb and Takeda
SO: none known.
LE: none known.
ST: none known.
KK: none known.
BS: none known.
AA: none known.
Figures








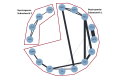


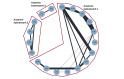


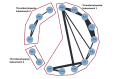


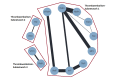





References
References to studies included in this review
Bahlis 2017 {published data only}
-
- Avet-Loiseau H, Hulin C, Benboubker L, Dimopoulos MA, Belch A, Reece D, et al. Impact of cytogenetics on outcomes of transplant-ineligible patients with newly diagnosed multiple myeloma treated with continuous lenalidomide plus low-dose dexamethasone in the first (MM-020) trial. In: Blood. Vol. 126. 2015:730.
-
- Bahlis N, Corso A, Mugge LO, Shen ZX, Desjardins P, Stoppa AM, et al. Impact of response in patients (pts) with stem cell transplant (SCT)-ineligible newly diagnosed multiple myeloma (NDMM) treated with continuous lenalidomide + low-dose dexamethasone (Rd) in the first trial. In: Clinical Lymphoma, Myeloma and Leukemia. Vol. 15. 2015:e56.
-
- Bahlis N, Corso A, Mugge Lo, Shen ZX, Desjardins P, Stoppa AM, et al. Assessing the benefit of continuous treatment in the first trial (MM-020): impact of response in patients with transplant-ineligible newly diagnosed multiple myeloma. In: Haematologica. Vol. 100. 2015:85-6.
-
- Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. New England Journal of Medicine 2014;371(10):906-17. - PubMed
Beksac 2011 {published data only}
-
- Beksac M, Haznedar R, Firatli-Tuglular T, Ozdogu H, Aydogdu I, Konuk N, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. European Journal of Haematology 2011;86(1):16-22. - PubMed
Durie 2017 {published data only}
-
- Durie B, Hoering A, Rajkumar SV, Abidi MH, Epstein J, Kahanic SP, et al. Bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (PTS) with previously untreated multiple myeloma without an intent for immediate Autologous Stem Cell Transplant (ASCT): results of the randomized phase III trial SWOG S0777. In: Blood. Vol. 126. 2015:25. - PMC - PubMed
-
- Durie BG, Hoering A Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet 2017;389:519-27. - PMC - PubMed
Facon 2007 {published data only}
-
- Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Harousseau JL, et al. Randomized clinical trial comparing melphalan-prednisone (MP), MP-thalidomide (MP-THAL) and high-dose therapy using melphalan 100 MG/Mý (MEL100) for newly diagnosed myeloma patients Aged 65-75 years. Interim analysis of the IFM 99-06 Trial on 350 patients. In: Blood. Vol. 104. 2004:63.
-
- Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet 2007;370(9594):1209-18. - PubMed
Hulin 2009 {published data only}
-
- Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. Journal of Clinical Oncology 2009;27(22):3664-70. - PubMed
-
- Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, et al. Melphalan-prednisone-thalidomide (MP-T) demonstrates a significant survival advantage in elderly patients >=75 years with multiple myeloma compared with melphalan-pPrednisone (MP) in a randomized, double-blind, placebo-controlled trial, IFM 01/01. In: Blood. Vol. 110. 2007.
-
- Hulin C, Virion J, Leleu X, Rodon P, Pegourie B, Benboubker L, et al. Comparison of melphalan-prednisone-thalidomide (MP-T) to melphalan-prednisone (MP) in patients 75 years of age or older with untreated multiple myeloma (MM). Preliminary results of the randomized, double-blind, placebo controlled IFM 01-01 trial. In: Journal of Clinical Oncology. Vol. 25. 2007:441.
Hungria 2016 {published data only}
-
- Anonymous. Iberoamerican protocol with thalidomide in patients with symptomatic newly diagnosed multiple myeloma over 65 years. available at: https://clinicaltrials.gov/show/nct01532856 (Accessed 06 May 2019).
-
- Crusoe E, Maiolino A, Bittencourt R, Fantl DB, MacIel J, Sampaio M, et al. A phase III study comparing thalidomide/cyclophosphamide/dexa vs thalidomide/dexa vs thalidomide/melphalan/prednisone in de novo multiple myeloma patients not eligible for ASCT. In: Blood. Vol. 118. 2011.
-
- Hungria V, Crusoe E, Maiolino A, Bittencourt R, Fantl D, Quero A, et al. A phase III study comparing thalidomide/cyclophosphamide/ dexa vs thalidomide/dexa vs thalidomide/melphalan/prednisone in de novo multiple myeloma patients not eligible for ASCT: preliminary analysis. In: Haematologica. Vol. 92. 2010:148.
-
- Hungria VT, Crusoe EQ, Maiolino A, Bittencourt R, Fantl D, Maciel JF, et al. Phase 3 trial of three thalidomide-containing regimens in patients with newly diagnosed multiple myeloma not transplant-eligible. Annals of Hematology 2016;95(2):271-8. - PubMed
Jacobus 2016 {published data only}
-
- Fonseca R, Rajkumar SV. Consolidation therapy with bortezomib/lenalidomide/ dexamethasone versus bortezomib/dexamethasone after a dexamethasone-based induction regimen in patients with multiple myeloma: a randomized phase III trial. Clinical Lymphoma and Myeloma 2008;8(5):315-7. - PubMed
-
- Jacobus SJ, Rajkumar SV, Weiss M, Stewart AK, Stadtmauer EA, Callander NS, et al. Randomized phase III trial of consolidation therapy with bortezomib-lenalidomide-Dexamethasone (VRd) vs bortezomib-dexamethasone (Vd) for patients with multiple myeloma who have completed a dexamethasone based induction regimen. Blood Cancer Journal 2016;6(7):e448. - PMC - PubMed
Katsuoka 2013 {published data only}
-
- Katsuoka Y, Kato Y, Omoto E, Sasaki O, Kimura H, Meguro K, et al. Phase ii trial of bortezomib based regimen for transplant-ineligible multiple myeloma-tomato study. In: Clinical Lymphoma, Myeloma and Leukemia. Vol. 13. 2013:S148.
Kim 2007 {published data only}
-
- Kim YK, Lee JJ, Sohn SK, Shin HJ, Lee SR, Shim HJ, et al. Efficacy and safety of thalidomide and dexamethasone combination with or without cyclophosphamide in patients with newly diagnosed multiple myeloma. In: Haematologica. Vol. 92. 2007:411-2.
Ludwig 2009 {published data only}
-
- Ludwig H, Drach J, Tothova E, Gisslinger H, Linkesch W, Jaksic B, et al. Thalidomide-dexamethasone (TD) versus melphalan-prednisolone (MP) as first line treatment in elderly patients with multiple myeloma (MM): an interim analysis. In: Onkologie. Vol. 28. 2005:14-5.
-
- Ludwig H, Hajek R, Tóthová E, Drach J, Adam Z, Labar B, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood 2009;113(15):3435-42. - PubMed
-
- Ludwig H, Tothova E, Hajek R, Drach J, Adam Z, Labar B, et al. Thalidomide-dexamethasone vs. melphalan-prednisone as first line treatment and thalidomide-interferon vs. interferon maintenance therapy in elderly patients with multiple myeloma. In: Blood. Vol. 110. 2007:163a.
-
- Ludwig H, Tothova E, Hajek R, Drach J, Labar B, Egyed M, et al. Thalidomide-dexamethasone versus melphalan-prednisolone as first line treatment in elderly patients with multiple myeloma: second interim analysis. In: Haematologica. Vol. 92. 2007:166.
-
- Ludwig HL, Drach J, Tothova E, Gisslinger H, Linkesch W, Jaksic B, et al. Thalidomide-dexamethasone versus melphalan-prednisolone as first line treatment in elderly patients with multiple myeloma: an interim analysis. In: European Hematology Association. 2005.
Magarotto 2016 {published data only}
-
- Bringhen S, Offidani M, Musto P, Liberati AM, Benevolo G, Cascavilla N, et al. Long term outcome of lenalidomide-dexamethasone (RD) vs melphalan-lenalidomide-prednisone (MPR) vs cyclophosphamide-prednisone-lenalidomide (CPR) as induction followed by. In: Blood. Vol. 130. 2017.
-
- Gay F, Bringhen S, Offidani M, Liberati A, Cellini C, Magarotto V, et al. Efficacy and safety of 3 lenalidomide-based combinations in elderly newly diagnosed multiple myeloma patients: results from the phase 3 community based emn01 trial. In: Haematologica. Vol. 98. 2013:95-6.
-
- Gentile M, Magarotto V, Offidani M, Musto P, Bringhen S, Teresa Petrucci M, et al. Lenalidomide and low-dose dexamethasone (Rd) versus bortezomib, melphalan, prednisone (VMP) in elderly newly diagnosed multiple myeloma patients: A comparison of two prospective trials. American Journal of Hematology 2017;92(3):244-50. - PubMed
-
- Larocca A, Bringhen S, Petrucci MT, Offidani M, Benevolo G, Galli M, et al. Early death in elderly patients with newly diagnosed multiple myeloma treated with new drugs: a pooled analysis of two randomized phase 3 trials. In: Haematologica. Vol. 100. 2015:51-2.
-
- Larocca A, Bringhen S, Petrucci MT, Offidani M, Paoli L, Pietrantuono G, et al. Early mortality in elderly newly diagnosed multiple myeloma patients treated with novel agents: a pooled analysis of two large randomized phase III trials. In: Haematologica. Vol. 100. 2015:81-2.
Mateos 2014 {published data only}
-
- Mateos MV, Oriol A, Martínez-López J, Gutiérrez N, Teruel AI, López de la Guía A, et al. Maintenance therapy with bortezomib plus thalidomide or bortezomib plus prednisone in elderly multiple myeloma patients included in the GEM2005MAS65 trial. In: Blood. Vol. 120. 2012:2581-8. - PubMed
-
- Mateos MV, Oriol A, Martínez-López J, Gutiérrez N, Teruel AI, Paz R, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncology 2010;11(10):934-41. - PubMed
-
- Mateos MV, Oriol A, Martínez-López J, Teruel AI, López de la Guía A, López J, et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: do we still need alkylators? Blood 2014;124(12):1887-93. - PubMed
-
- Mateos MV, Oriol A, Martinez-Lopez J, Gutierrez N, Teruel AI, Paz R, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncology 2010;11(10):934-41. - PubMed
-
- Mateos MV, Oriol A, Martinez-Lopez J, Teruel AI, Lopez de la Guia A, Blanchard MJ, et al. Do we still need the alkylators as part of the up front treatment of elderly newly diagnosed multiple myeloma patients? Updated follow-up of GEM2005MAS65 Spanish trial comparing VMP vs VTP as induction. In: Haematologica. Vol. 99. 2014:221.
Mookerjee 2017 {published data only}
-
- Mookerjee A, Gupta R, Jasrotia S, Sahoo R, Kumar R, Thulkar S, et al. Bortezomib, lenalidomide and low-dose dexamethasone (VRD) versus lenalidomide and low-dose dexamethasone (LD) for newly-diagnosed multiple myeloma-a randomized phase III study. In: Blood. Vol. 130. 2017.
Morgan 2011 {published data only}
Niesvizky 2015 {published data only}
-
- Moon MA. Triple therapy added toxicity without survival benefit in multiple myeloma. Journal of Clinical Oncology 2015;11(7):3-4.
-
- Niesvizky R, Flinn I, Rifkin R, Charu V, Essell J, Gaffar Y, et al. Efficacy and safety of three bortezomib-based induction regimens followed by weekly bortezomib maintenance therapy in newly diagnosed multiple myeloma (MM) patients (PTS) ineligible for transplant: results of the phase 3B upfront study. In: Haematologica. Vol. 96. 2011:S98.
-
- Niesvizky R, Flinn I, Rifkin R, Gabrail N, Charu V, Clowney B, et al. Patient-reported quality of life (QOL) in previously untreated, elderly multiple myeloma (MM) patients treated with bortezomib-based regimens: results from the phase 3B upfront study. In: Haematologica. Vol. 96. 2011:123-4.
-
- Niesvizky R, Flinn IW, Rifkin R, Gabrail N, Charu V, Clowney B, et al. Community-based phase IIIB trial of three UPFRONT bortezomib-based myeloma regimens. Journal of Clinical Oncology 2015;33(33):3921-9. - PubMed
-
- Niesvizky R, Flinn IW, Rifkin R, Gabrail N, Charu V, Clowney B. Patient-reported quality of life (QOL) in elderly, newly diagnosed multiple myeloma (MM) patients receiving bortezomib-based combinations: results from all randomized patients in the community-based, phase 3b UPFRONT study. In: Blood. Vol. 118. 2011.
Palumbo 2006 {published data only}
-
- Palumbo A, Bringhen S, Caravita T, Callea V, Zambello R, Pregno P, et al. A prospective randomized controlled trial of oral melphalan, prednisone, thalidomide versus oral melphalan, prednisone in elderly newly diagnosed myeloma patients. In: Haematologica. Vol. 91. 2006.
-
- Palumbo A, Bringhen S, Caravita T, Falcone A, Callea V, Cangialosi C, et al. Oral melphalan, prednisone, thalidomide versus oral melphalan, prednisone in elderly newly diagnosed myeloma patients. In: Haematologica. Vol. 92. 2007:147.
-
- Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet 2006;367(9513):825-31. - PubMed
-
- Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone and thalidomide in elderly multiple myeloma patients: updated results of a randomized controlled trial. In: Haematologica. Vol. 93. 2008:363-5. - PubMed
-
- Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. In: Blood. Vol. 112. 2008:3107-14. - PubMed
Palumbo 2012 {published data only}
-
- Cavo M, Dimopoulos M, Palumbo A, Weisel K, Delforge M, Blade J, et al. Impact of renal impairment on the efficacy and safety of melphalan-prednisone-lenalidomide (LEN) induction followed by LEN maintenance in newly diagnosed multiple myeloma: mM-015 post-hoc analysis. In: Haematologica. Vol. 98. 2013:330-1.
-
- Dimopoulos MA, Delforge M, Hájek R, Kropff M, Petrucci MT, Lewis P, et al. Lenalidomide, melphalan, and prednisone, followed by lenalidomide maintenance, improves health-related quality of life in newly diagnosed multiple myeloma patients aged 65 years or older: results of a randomized phase III trial. Haematologica 2013;98(5):784-8. - PMC - PubMed
-
- Dimopoulos MA, Palumbo A, Hajek R, Kropff M, Petrucci MT, Lewis P, et al. Factors that influence health-related quality of life in newly diagnosed patients with multiple myeloma aged? 65 years treated with melphalan, prednisone and lenalidomide followed by lenalidomide maintenance: results of a randomized trial. Leukemia and Lymphoma 2014;55(7):1489-97. - PMC - PubMed
-
- Gay F, Cavallo F, Raimondo F, Hardan I, Nagler A, Petrucci MT, et al. Impact of continuous treatment vs fixed duration of therapy in newly diagnosed myeloma patients: pFS1, PFS2, OS endpoints. In: Haematologica. Vol. 99. 2014:519.
-
- Palumbo A, Adam Z, Kropff M, Foa R, Catalano J, Gisslinger H. A phase 3 study evaluating the efficacy and safety of lenalidomide (len) combined with melphalan and prednisone followed by continuous lenalidomide maintenance (MPR-R) in patients (pts) = 65 Years (yrs) with newly diagnosed multiple myeloma (NDMM): updated results for pts aged 65-75 yrs enrolled in MM-015. In: Blood. Vol. 118. 2011.
Palumbo 2014 {published data only}
-
- Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood 2010;116(23):4745-53. - PubMed
-
- Bringhen S, Larocca A, Rossi D, Romano A, Genuardi M, Ria R, et al. Efficacy and safety of once weekly bortezomib in multiple myeloma patients. In: Blood. Vol. 116. 2010. - PubMed
-
- Bringhen S, Magarotto V, Rossi D, Ria R, Offidani M, Patriarca F, et al. Bortezomib, melphalan, prednisone and thalidomide followed by maintenance with bortezomib and thalidomide (VMPT-VT) vs bortezomib, melphalan, prednisone (VMP) alone as first line treatment of multiple myeloma: updated follow-up and impact of prognostic factors. In: Haematologica. Vol. 96. 2011:67-8.
-
- Corso A, Raimondo F, Mangiacavalli S, Ria R, Offidani M, Pascutto C, et al. Bortezomib-based therapy overcomes the prognostic role of ISS score in multiple myeloma patients not eligible for transplant. In: Haematologica. Vol. 96. 2011:128.
-
- Gay F, Cavallo F, Raimondo F, Hardan I, Nagler A, Petrucci MT, et al. Impact of continuous treatment vs fixed duration of therapy in newly diagnosed myeloma patients: pFS1, PFS2, OS endpoints. In: Haematologica. Vol. 99. 2014:519.
Pawlyn 2017 {published data only}
-
- Brioli A, Davies F, Gregory W, Hinsley S, Marshall S, Pawlyn C, et al. Low rate of secondary primary malignancies (SPMs) in newly diagnosed multiple myeloma (MM) patients treated with lenalidomide: first results from the MRC MM XI trial. In: Haematologica. Vol. 98. 2013:104.
-
- Jackson GH, Davies FE, Pawlyn C, Cairns DA, Striha A, Collett C, et al. Lenalidomide is a highly effective maintenance therapy in myeloma patients of all ages; Results of the phase III myeloma XI study. In: Blood. Vol. 128. 2016.
-
- Jones JR, Cairns D, Gregory W, Sigsworth R, Collett C, Pawlyn C, et al. The NCRI Myeloma XI trial for newly diagnosed symptomatic multiple myeloma (NDMM); second primary malignancy (SPM) incidence when lenalidomide is used as an induction and maintenance treatment option. In: British Journal of Haematology. Vol. 173. 2016:86.
-
- Jones JR, Cairns DA, Sigsworth R, Collett C, Pawlyn C, Striha A, et al. Myeloma XI trial for newly diagnosed multiple myeloma (NDMM); a report of second primary malignancy (SPM) rates and the importance of review of reported cases. In: Blood. Vol. 126. 2015:1847.
Sacchi 2011 {published data only}
-
- Sacchi S, Marcheselli R, Lazzaro A, Morabito F, Fragasso A, Di Renzo N, et al. A randomized trial with melphalan and prednisone versus melphalan and prednisone plus thalidomide in newly diagnosed multiple myeloma patients not eligible for autologous stem cell transplant. Leukemia and Lymphoma 2011;52(10):1942-8. - PubMed
San Miguel 2008 {published data only}
-
- Delforge M, Terpos E, Richardson PG, Shpilberg O, Khuageva NK, Schlag R, et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. European Journal of Haematology 2011;86(5):372-84. - PubMed
-
- Dimopoulos MA, Mateos MV, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, et al. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: subanalysis of the phase 3 VISTA study. European Journal of Haematology 2011;86(1):23-31. - PubMed
-
- Dimopoulos MA, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, Kastritis E, et al. VMP (Bortezomib, Melphalan, and Prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. Journal of Clinical Oncology 2009;27(36):6086-93. - PubMed
-
- Harousseau JL, Palumbo A, Richardson PG, Schlag R, Dimopoulos MA, Shpilberg O, et al. Superior outcomes associated with complete response in newly diagnosed multiple myeloma patients treated with nonintensive therapy: analysis of the phase 3 VISTA study of bortezomib plus melphalan-prednisone versus melphalan-prednisone. Blood 2010;116(19):3743-3750. - PubMed
-
- Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. Journal of Clinical Oncology 2010;28(13):2259-66. - PubMed
Stewart 2015 {published data only}
-
- Stewart AK, Jacobus S, Fonseca R, Weiss M, Callander NS, Chanan Khan AA, et al. E1A06: a phase III trial comparing melphalan, prednisone, and thalidomide (MPT) versus melphalan, prednisone, and lenalidomide (MPR) in newly diagnosed multiple myeloma MM). In: Haematologica. Vol. 99. 2014:220.
Waage 2010 {published data only}
-
- Waage A, Gimsing P, Fayers P, Abildgaard N, Ahlberg L, Björkstrand B, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood 2010;116(9):1405-12. - PubMed
-
- Waage A, Gimsing P, Juliusson G, Turesson I, Fayers P. Melphalan-prednisone-thalidomide to newly diagnosed patients with multiple myeloma: a placebo controlled randomised phase 3 trial. In: Blood. Vol. 110. 2007:32a.
Wijermans 2010 {published data only}
-
- Beurden-Tan C, Blommestein H, Zweegman S, Sonneveld P. The relationship of response on time to next treatment based on evidence from two RCTs in newly diagnosed stem cell transplantation ineligible multiple myeloma patients. In: Blood. Vol. 128. 2016:(no pagination).
-
- Verelst S, Termorshuizen F, Uyl-De Groot C, Sonneveld P, Wijermans P. Effect of thalidomide with melphalan and prednisone on health-related quality of life (HRQOL) in elderly patients with newly diagnosed multiple myeloma: a prospective analysis in a randomized trial (HOVON 49). In: Haematologica. Vol. 96. 2011:S135. - PubMed
-
- Verelst S, Termorshuizen F, Uyl-de Groot C, Schaafsma MR, Ammerlaan R, Wittebol S. Effect of thalidomide with melphalan and prednisone on health-related quality of life (HRQOL) in elderly patients with newly diagnosed multiple myeloma: a prospective analysis in a randomized trial. In: Blood. Vol. 116. 2010. - PubMed
-
- Verelst SG, Termorshuizen F, Uyl-de Groot CA, Schaafsma MR, Ammerlaan AH, Wittebol S, et al. Effect of thalidomide with melphalan and prednisone on health-related quality of life (HRQoL) in elderly patients with newly diagnosed multiple myeloma: a prospective analysis in a randomized trial. Annals of Hematology 2011;90(12):1427-39. - PubMed
-
- Wijermans P, Schaafsma M, Norden Y, Ammerlaan R, Sonneveld P, Wittebol S, et al. Melphalan + prednisone vs melphalan + prednisone + thalidomide in induction therapy for multiple myeloma in elderly patients: first interim results of the Dutch cooperative group HOVON. In: Haematologica. Vol. 93. 2008:177.
Zweegman 2016 {published data only}
-
- Beurden-Tan C, Blommestein H, Zweegman S, Sonneveld P. The relationship of response on time to next treatment based on evidence from two RCTs in newly diagnosed stem cell transplantation ineligible multiple myeloma patients. In: Blood. Vol. 128. 2016:(no pagination).
-
- Kongsgaard Nielsen L, Stege C, Witte B, Holt B, Mellqvist UH, Salomo M, et al. Health-related quality of life in non-transplant eligible newly diagnosed multiple myeloma patients treated with melphalan/prednisolone plus either thalidomide or lenalidomide; results of the HOVON87/NMSG18 study. In: Quality of Life Research. Vol. 26. 2017:56-7.
-
- Stege C, Holt B, Mellqvist UH, Levin MD, Salomo M, Abildgaard N, et al. Frailty predicts survival and toxicity in newly diagnosed multiple myeloma patients ineligible for autologous stem cell transplantation; report of the HOVON-87/NMSG-18 study group. In: Blood. Vol. 130. 2017:(no pagination).
-
- Stege C, Kongsgaard Nielsen L, Witte B, Holt B, Mellqvist UH, Salomo M, et al. Quality of life with melphalan/prednisone plus either thalidomide (MPT-T) or lenalidomide (MPR-R) in non-transplant eligible newly diagnosed multiple myeloma; Results of the Hovon87/NMSG18 study. In: Haematologica. Vol. 102. 2017:190.
-
- Zweegman S, Holt B, Mellqvist UH, Salomo M, Bos GM, Levin MD, et al. Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood 2016;127(9):1109-16. - PubMed
References to studies excluded from this review
Anonymous 2003 {published data only}
-
- Anonymous. A multicenter parallel-group, placebo controlled, randomized, double-blind study of combination thalidomide plus glucocorticoid therapy versus glucocorticoid therapy alone as induction therapy for previously untreated subjects with multiple myeloma. available at: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-00500364/... (accessed 06 May 2019).
Barlogie 2004 {published data only}
-
- Anonymous. Study of bortezomib and Revlimid ™ for patients relapsing or progressing on total therapy II. available at: https://clinicaltrials.gov/show/nct00093028 (Accessed 28 May 2019).
-
- Barlogie B, Rasmussen E, Tricot G, Crowley J. Management of patients with multiple myeloma (MM) failing total therapy 2 (TT 2) according to thalidomide (THAL) randomization. In: Blood. Vol. 104. 2004:414a.
Brioli 2014 {published data only}
-
- Brioli A, Galli M, Tacchetti P, Crippa C, Spadano T, Pezzi A, et al. A combination therapy with bortezomib (BOR) and thalidomide in newly diagnosed myeloma patients is associated with a low incidence of second primary malignancies (SPMS). In: Haematologica. Vol. 99. 2014:373.
Facon 2006 {published data only}
-
- Facon T, Mary JY, Attal M, Pegourie B, Harousseau JL, Sadoun A. Melphalan-prednisone versus dexamethasone-based regimens for newly diagnosed myeloma patients aged 65-75 years. Final analysis of the IFM 95-01 trial on 489 patients. In: Blood. Vol. 102. 2003:147a.
-
- Facon T, Mary JY, Pégourie B, Attal M, Renaud M, Sadoun A, et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood 2006;107(4):1292-8. - PubMed
Facon 2017 {published data only}
-
- Anonymous. Phase 3 study of carfilzomib, melphalan, prednisone vs bortezomib, melphalan, prednisone in newly diagnosed multiple myeloma. available at: ttps://clinicaltrials.gov/show/nct01818752 (accessed 03 July 2019).
-
- Facon T, Lee JH, Moreau P, Niesvizky R, Dimopoulos MA, Hajek R, et al. Phase 3 Study (CLARION) of carfilzomib, melphalan, prednisone (KMP) v bortezomib, melphalan, prednisone (VMP) in newly diagnosed multiple myeloma (NDMM). Clinical Lymphoma, Myeloma and Leukemia 2017;17(1):e26-7.
Facon 2018 {published data only}
-
- Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. LBA-2 phase 3 randomized study of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) in patients with newly diagnosed multiple myeloma (NDMM) ineligible for transplant (MAIA). In: Blood. Vol. 132. 2018:LBA-2.
Foa 2007 {published data only}
-
- Foa R, Weber D, Dimopoulos M, Olesnyckyj M, Yu Z, Zeldis J, et al. Lenalidomide/dexamethasone improves response and prolongs time to progression, even in patients with IgA multiple myeloma: a sub-analysis of the MM-009/010 studies. In: Blood. Vol. 110. 2007:284b.
Harousseau 2003 {published data only}
-
- Harousseau JL, Attal M. The IFM-90 trial. In: Hematology Journal. Vol. 4. 2003:S55.
Hejlova 2000 {published data only}
-
- Hejlova N, Hajek R, Adam Z, Scudla Z, Bacovsky J, Koza V, et al. Report of the "4W" trial of the Czech myeloma group. In: Hematology Journal. Vol. 1. 2000:168-9.
Hernández 2004 {published data only}
-
- Hernández JM, García-Sanz R, Golvano E, Bladé J, Fernandez-Calvo J, Trujillo J, et al. Randomized comparison of dexamethasone combined with melphalan versus melphalan with prednisone in the treatment of elderly patients with multiple myeloma. British Journal of Heamatology 2004;127(2):159-64. - PubMed
Kumar 2012 {published data only}
-
- Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012;119(19):4375-82. - PubMed
Ludwig 2017a {published data only}
-
- Anonymous. Patients with newly diagnosed multiple myeloma comparing KTd vs. KRd induction therapy and investigating a K-mono maintenance strategy. Available at: https://clinicaltrials.gov/show/nct02891811 (accessed 03 July 2019).
-
- Ludwig H. AGMT-MM2 A randomized phase II study in transplant ineligible patients with newly diagnosed multiple myeloma (NDMM) comparing Carfilzomib + Thalidomide + Dexamethasone(KTd) with Carfilzomib + Lenalidomide + Dexamethasone (KRd) induction therapy followed by Carfilzomib (K) maintenance or control. In: Magazine of European Medical Oncology. Vol. 10. 2017:S44-s45.
Mateos 2016 {published data only}
-
- Mateos MV, Gutierrez NC, Martin ML, Martinez-Lopez J, Hernandez MT, Martinez R, et al. Bortezomib plus melphalan and prednisone (VMP) followed by lenalidomide and dexamethasone (RD) in newly diagnosed elderly myeloma patients overcome the poor prognosis of high-risk cytogenetic abnormalities (CA) detected by fluorescence in situ hibridization (FISH). In: Blood. Vol. 126. 2015:4243.
-
- Mateos MV, Martínez-López J, Hernández MT, Ocio EM, Rosiñol L, Martínez R, et al. Sequential vs alternating administration of VMP and Rd in elderly patients with newly diagnosed MM. Blood 2016;127(4):420-5. - PubMed
-
- Mateos MV, Martinez-Lopez J, Hernandez M, Martinez R, Rosinol L, Ocio EM, et al. Depth of response as surrogate marker for progression-free survival and overall survival in elderly newly diagnosed myeloma patients treated with VMP and Rd: gEM2010MAS65. In: Haematologica. Vol. 102. 2017:143.
-
- Mateos MV, Martinez-Lopez J, Hernandez MT, Ocio EM, Rosinol L, Martinez R, et al. Bortezomib, melphalan, prednisone (VMP) and lenalidomide plus dexamethasone (RD) is the optimal combination for patients with newly diagnosed multiple myeloma (MM) patients between 65 and 80 years. In: Blood. Vol. 126. 2015:1848.
Mateos 2018 {published data only}
-
- Facon T, Cavo M, Jakubowiak A, San Miguel J, Kumar S, Orlowski RZ, et al. Two randomized open-label studies of daratumumab (DARA) plus standard of care treatment versus standard of care alone in patients with previously untreated multiple myeloma (MM) ineligible for high-dose therapy: 54767414MMY3007 (Alcyone) and 54767414MMY3008 (Maia). In: Clinical Lymphoma, Myeloma and Leukemia. Vol. 15. 2015:e294-5.
-
- Mateos MV, Cavo M, Jakubowiak AJ, Carson RL, Qi M, Bandekar R, et al. A randomized open-label study of bortezomib, melphalan, and prednisone (VMP) versus daratumumab (DARA) plus VMP in patients with previously untreated multiple myeloma (MM) who are ineligible for high-dose therapy: 54767414MMY3007 (Alcyone). In: Journal of Clinical Oncology. Vol. 33. 2015:supplement 1 (no pagination).
-
- Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus bortezomib, melphalan, and prednisone (D-VMP) versus bortezomib, melphalan, and prednisone (VMP) in newly diagnosed multiple myeloma (NDMM) patients (PTS) ineligible for transplant: a phase 3 ran-domized study (alcyone). In: Haematologica. Vol. 103. 2018:22.
-
- Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. New England Journal of Medicine 2018;378(6):518-28. - PubMed
-
- Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak AJ, Knop S, et al. Phase 3 randomized study of daratumumab plus bortezomib, melphalan, and prednisone (D-VMP) versus bortezomib, melphalan, and prednisone (VMP) in newly diagnosed multiple myeloma (NDMM) patients (Pts) ineligible for transplant (ALCYONE). In: Blood. Vol. 130. 2017:Supplement 1 (no pagination).
Merz 2015 {published data only}
-
- Merz M, Salwender HJ, Haenel M, Bertsch U, Kunz C, Hielscher T, et al. Clinical risk factors for peripheral neuropathy in patients treated with subcutaneous or intravenous bortezomib for newly diagnosed multiple myeloma. In: BLood. Vol. 126. 2015:4233.
Montefusco 2013 {published data only}
-
- Montefusco V. First-line treatment in elderly patients. In: European journal of cancer. Vol. 49. 2013:S19.
Morgan 2002 {published data only}
-
- Morgan G, Davies FE, Hawkins K, Brown J, Drayson MT. The MRC Myeloma VII Trial of standard versus intensive treatment in patients <65 years of age with multiple myeloma. In: Blood. Vol. 100. 2002:178a.
NCT00522392 {published data only}
-
- Anonymous. Bortezomib and dexamethasone with or without lenalidomide in treating patients with multiple myeloma previously treated with dexamethasone. available at: https://clinicaltrials.gov/show/nct00522392 (accessed 06 May 2019).
NCT00734877 {published data only}
-
- Anonymous. UARK 2013-13, Total Therapy 4B - Formerly 2008-01 - A phase III trial for low risk myeloma. available at: https://clinicaltrials.gov/show/nct00734877 (accessed 06 May 2019).
NCT01850524 {published data only}
-
- Anonymous. IXAZOMIB plus lenalidomide and dexamethasone versus placebo plus lenalidomide and dexamethasone in adult patients with newly diagnosed multiple myeloma. available at: https://clinicaltrials.gov/show/nct01850524 (accessed 03 July 2019).
NCT01863550 {published data only}
-
- Anonymous. Bortezomib or carfilzomib With lenalidomide and dexamethasone in treating patients with newly diagnosed multiple myeloma. available at: https://clinicaltrials.gov/show/nct01863550 (accessed 03 July 2019).
NCT02586038 {published data only}
-
- Anonymous. Study that compares 3 arm: MLN9708 dexamethasone, MLN9708 cyclophosphamide and dexamethasone, MLN9708 thalidomide and dexamethasone followed by maintenance with MLN9708 in newly diagnosed elderly multiple myeloma patients. available at: https://clinicaltrials.gov/show/nct02586038 (accessed 03 July 2019).
NCT03710603 {published data only}
-
- Anonymous. A study comparing daratumumab, VELCADE (bortezomib), lenalidomide, and dexamethasone (D-VRd) with VELCADE, lenalidomide, and dexamethasone (VRd) in participants with untreated multiple myeloma and for whom hematopoietic stem cell transplant is not planned as initial therapy. available at: https://clinicaltrials.gov/show/nct03652064 (accessed 03 July 2019).
-
- Anonymous. Daratumumab, VELCADE (bortezomib), lenalidomide and dexamethasone compared to VELCADE, lenalidomide and dexamethasone in subjects with previously untreated multiple myeloma. available at: https://clinicaltrials.gov/show/nct03710603 (accessed 03 July 2019).
Niesvizky 2003 {published data only}
-
- Niesvizky R, Pekle K, Lyons L, Pearse RN, Bergsagel PL, Schuster MW. Dexamethsone alone, or in combination with low-dose thalidomide as induction therapy for advanced multiple myeloma, and the effect of the addition of clarithromycin (Biaxin?) on response rate. Interim results of a prospective, sequential, randomized trial. In: Blood. Vol. 102. 2003:237a.
Offidani 2004 {published data only}
-
- Offidani M, Corvatta L, Marconi M, Olivieri A, Catarini M, Mele A, et al. Thalidomide plus oral melphalan compared with thalidomide alone for advanced multiple myeloma. Hematology Journal 2004;5(4):312-7. - PubMed
Palumbo 2016 {published data only}
-
- Lonial S, Ribeiro De Oliveira M, Yimer H, Mateos MV, Rifkin R, Schjesvold F, et al. KEYNOTE-185: a randomized, open-label phase 3 study of pembrolizumab in combination with lenalidomide and lowdose dexamethasone in newly diagnosed and treatmentnaive multiple myeloma (MM). In: Annals of Oncology. Vol. 27. 2016:viii16.
-
- Palumbo A, Mateos MV, San Miguel J, Shah J, Thompson S, Marinello P, et al. Pembrolizumab in combination with lenalidomide and low-dose dexamethasone in newly diagnosed and treatment-naive multiple myeloma (MM): randomized, phase 3 KEYNOTE-185 study. In: Annals of Oncology. Vol. 27. 2016:(no pagination).
-
- Shah J, Lonial S, Oliveira MR, Yimer H, Mateos MV, Rifkin R, et al. KEYNOTE-185: randomized, open-label, phase III study of pembrolizumab in combination with lenalidomide and low-dose dexamethasone in patients with newly diagnosed and treatment-naive multiple myeloma (MM). In: Journal for Immunotherapy of Cancer. Vol. 4. 2016.
Rajkumar 2006b {published data only}
-
- Greipp PR. Eastern Cooperative Oncology Group E1A00: phase III randomized study of dexamethasone with or without thalidomide in patients with newly diagnosed multiple myeloma. Clinical Advances in Hematology & Ooncology 2003;1(3):188-9. - PubMed
-
- Rajkumar SV, Blood E , Vesole D, Fonseca R, Greipp PR, Eastern Cooperative Oncology Group. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2006;24(3):431-6. - PubMed
-
- Rajkumar SV, Vesole DH, Shepard PR, Greipp PR. A randomised phase III trial of thalidomide plus dexamethasone versus dexamethasone in newly diagnosed multiple myeloma (E1A00): a trial coordinated by the Eastern Cooperative Oncology Group. In: Journal of Clinical Oncology. Vol. 22. 2004:558. - PubMed
-
- Rajkumar SV, Vesole DH, Shepard R, Greipp PR. Thalidomide plus dexamethasone versus dexamethasone alone in newly diagnosed multiplemMyeloma (E1A00): results of a phase III trial coordinated by the Eastern Cooperative Oncology Group. In: Blood. Vol. 104. 2004:63.
Rajkumar 2008 {published data only}
-
- Rajkumar SV, Hussein M, Catalano J, Jedrzejcak W, Sirkovich S, Olesnyckyj M, et al. A multicenter, randomized, double-blind, placebo-controlled trial of thalidomide plus dexamethasone versus dexamethasone alone as initial therapy for newly diagnosed multiple myeloma. In: Journal of Clinical Oncology. Vol. 24. 2006:426. - PMC - PubMed
-
- Rajkumar SV, Hussein M, Catalano J, Jedrzejczak W, Sirkovich S, Olesnyckyj M, et al. A randomized, double-blind, placebo-controlled trial of thalidomide plus dexamethasone versus dexamethasone alone as primary therapy for newly diagnosed multiple myeloma. In: Blood. Vol. 108. 2006:238.
-
- Rajkumar SV, Rosiñol L, Hussein M, Catalano J, Jedrzejczak W, Lucy L, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. Journal of Clinical Oncology 2008;26(13):2171-7. - PMC - PubMed
San Miguel 2014 {published data only}
-
- San Miguel J, Blade J, Samoilova O, Shpilberg O, Grosicki S, Maloisel F, et al. Randomized, open-label, phase 2 study of siltuximab (an anti-il-6 mab) and bortezomib-melphalan-prednisone versus bortezomib-melphalan-prednisone in patients with previously untreated multiple myeloma. In: Haematologica. Vol. 98. 2013:97.
Takezako 2017 {published data only}
-
- Anonymous. PH III study of lenalidomide and dexamethasone with or without elotuzumab to treat peviously untreated multiple myeloma (ELO 1 substudy). available at: https://clinicaltrials.gov/show/nct01891643 (accessed 03 July 2019).
-
- Anonymous. Phase III study of lenalidomide and dexamethasone with or without elotuzumab to treat newly diagnosed, previously untreated multiple myeloma. available at: https://clinicaltrials.gov/show/nct01335399 (accessed 03 July 2019).
-
- Takezako N, Ohta K, Handa H, Hori M, Kinoshita G, Shelat S, et al. Elotuzumab plus lenalidomide/dexamethasone (ELD) vs LD in patients with newly diagnosed multiple myeloma: phase 2, randomized, open-label study in Japan. In: Blood. Vol. 130. 2017:supplement 1 (no pagination).
Usmani 2014 {published data only}
-
- Anonymous. S1211 Bortezomib, Dexamethasone, and Lenalidomide With or Without Elotuzumab in Treating Patients With Newly Diagnosed High-Risk Multiple Myeloma. available at: https://clinicaltrials.gov/show/nct01668719 (accessed 03 July 2019).
-
- Usmani SZ, Hoering A, Sexton R, Ailawadhi S, Shah JJ, Fredette S, et al. SWOG 1211: a randomized phase I/II study of optimal induction therapy for newly diagnosed high-risk multiple myeloma (HRMM). In: Journal of Clinical Oncology. Vol. 32. 2014:15 suppl.1 (no pagination).
White 2007 {published data only}
-
- White DJ, Kovacs MJ, Belch A, Stewart K, Chen C, Rubin S, et al. Phase II testing of lenalidomide plus melphalan for previously untreated older patients with multiple myeloma: toxicity data from the NCIC CTG MY.11 trial. In: Blood. Vol. 110. 2007:63a.
Zonder 2010 {published data only}
-
- Zonder JA, Crowley J, Hussein MA, Bolejack V, Moore DF, Whittenberger BF, et al. Superiority of lenalidomide (Len) plus high-dose dexamethasone (HD) compared to HD alone as treatment of newly-diagnosed multiple myeloma (NDMM): results of the randomized, double-blinded, placebo-controlled SWOG trial S0232. In: Blood. Vol. 110. 2007:32a.
-
- Zonder JA, Crowley JJ, Bolejack V, Hussein MA, Moore DF, Whittenberger BF. A randomized Southwest Oncology Group study comparing dexamethasone (D) to lenalidomide + dexamethasone (LD) as treatment of newly-diagnosed multiple myeloma (NDMM): impact of cytogenetic abnormalities on efficacy of LD, and updated overall study results. In: Journal of Clinical Oncology. Vol. 26. 2008:459.
-
- Zonder JA. Phase III randomized study of dexamethasone with or without CC-5013 in patients with newly diagnosed multiple myeloma. http://cochranelibrary-wiley.com/o/cochrane/clcentral/articles/578/CN-00... 2003.
Additional references
Ailawadhi 2018
Anderson 2011
-
- Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annual Review of Pathology 2011;6:249-74. - PubMed
Anderson 2015
Argyriou 2008
-
- Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood 2008;112(5):1593-9. - PubMed
Asatsuma‐Okumura 2019
-
- Asatsuma-Okumura T, Ito T, Handa H. Molecular mechanisms of cereblon-based drugs. Pharmacology and Therapeutics 2019;202:132-9. - PubMed
Bianchi 2015
Blommestein 2019
Cancer Research UK 2018
-
- Cancer Research UK . Myeloma statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... (accessed 27 August 2018).
Chaimani 2017
-
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;83:65-74. - PubMed
Covidence systematic review software [Computer program]
-
- Veritas Health Innovation Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, Available at www.covidence.org.
Cowan 2018
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses on behalf of the Cochrane Statistical Methods Group. In: Higgins JP Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dias 2010
-
- Dias S, Welten NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis.. Statistics in Medicine 2010;29(7-8):932-44. [PMID: ] - PubMed
Egger 1997
Fayers 2011
-
- Fayers P M, Palumbo A, Hulin C, Waage A, Wijermans P, Beksac M, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood 2011;118(5):1239-47. - PubMed
Fink 2015
Gandolfi 2017
-
- Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG. The proteasome and proteasome inhibitors in multiple myeloma. Cancer and Metastasis Reviews 2017;36(4):561-84. - PubMed
Higgins 2011a
-
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JP Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Altman DG, Sterne JA (editors. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org..
Higgins 2011c
-
- Higgins JP, Deeks JJ, Altman DG (editors. Chapter 16: Special topics in statistics. In: Higgins JP Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holstein 2017
IMS Institute for Healthcare Informatics 2015
-
- IMS Institute for Healthcare Informatics. Understanding the Role and Use of Essential Medicines Lists,. Available at: http://apps.who.int/medicinedocs/documents/s21980en/s21980en.pdf (accessed 30 August 2019) 2015.
Key 2019
Kimpton 2016
-
- Kimpton M, Buyting R, Corsi DJ, Rupani N, Carrier M, McCurdy A. Incidence and risk factors of venous thromboembolism in patients with multiple myeloma receiving immunomodulatory agents. Blood 2016;128(22):2624.
Kirtane 2017
Kuehl 2012
Kuhr 2016
-
- Kuhr K, Wirth D, Srivastava K, Lehmacher W, Hellmich M. First-line therapy for non-transplant eligible patients with multiple myeloma: direct and adjusted indirect comparison of treatment regimens on the existing market in Germany. European Journal of Clinical Pharmacology 2016;72(3):257-65. - PubMed
Kumar 2018
-
- Kumar SK, Callander NS, Alsina M, Atanackovic D, Biermann JS, Castillo J, et al. NCCN guidelines insights: multiple myeloma, version 3.2018. Journal of the National Comprehensive Cancer Network 2018;16(1):11-20. - PubMed
Liu 2017
Lu 2014
Ludwig 2017b
McMaster 2015 [Computer program]
-
- GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc). Available from www.gradepro.org, 2015.
Miceli 2008
-
- Miceli T, Colson K, Gavino M, Lilleby K. Myelosuppression associated with novel therapies in patients with multiple myeloma: consensus statement of the IMF Nurse Leadership Board. Clinical Journal of Oncology Nursing 2008;12(Supplement 3):13-20. - PubMed
Moher 2009
Moreau 2012
Moreau 2017
-
- Moreau P, San Miguel J, Sonneveld P, Mateos MV, Zamagni E, Avet-Loiseau H, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2017;28 Suppl 4:iv52-61. - PubMed
National Cancer Institute 2018
-
- National Cancer Institute. Cancer stat facts: myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html (accessed 27 August 2018).
Nwabuko 2017
Palumbo 2004
-
- Palumbo A, Bringhen S, Petrucci MT, Musto P, Rossini F, Nunzi M, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood 2004;104(10):3052-7. - PubMed
Palumbo 2008
-
- Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia 2008;22(2):414-23. - PubMed
Palumbo 2011
-
- Palumbo A, Anderson K. Multiple myeloma. New England Journal of Medicine 2011;364(11):1046-60. - PubMed
Palumbo 2013
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. - PubMed
Puhan 2014
-
- Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (Clinical research ed.) 2014;349:g5630. - PubMed
Quach 2010
R 2018 [Computer program]
-
- R Foundation for Statistical Computing R: A language and environment for statistical computing. R Core Team. Vienna, Austria: R Foundation for Statistical Computing, 2018.
Rajkumar 2014
-
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncology 2014;15(12):e538-48. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richardson 2018
-
- Richardson PG, Laubach J, Gandolfi S, Facon T, Weisel K, O'Gorman P. Maintenance and continuous therapy for multiple myeloma. Expert Review of Anticancer Therapy 2018;18(8):751-64. - PubMed
Rücker 2012
-
- Rücker G. Network meta-analysis, electrical networks and graph theory. Research Synthesis Methods 2012;3(4):312-24. - PubMed
Rücker 2014
-
- Rücker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi-arm studies in network meta-analysis. Statistics in Medicine 2014;33(25):4353-69. - PubMed
Rücker 2015
Rücker 2018 [Computer program]
-
- http://CRAN.R-project.org/package=netmeta netmeta: Network Meta-Analysis using Frequentist Methods. R package version 0.9-8. Rücker G, Schwarzer G, Krahn U, König J. http://CRAN.R-project.org/package=netmeta, 2018.
San Miguel 2013
-
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. Journal of Clinical Oncology 2013;31(4):448-55. - PubMed
Schwarzer 2015
-
- Schwarzer G, Carpenter JR, Rücker G. Chapter 8: Network Meta-Analysis. In: netmeta: Network Meta-Analysis using Frequentist Methods. Springer International Publishing Switzerland, 2015.
Schwarzer 2018 [Computer program]
-
- http://CRAN.R-project.org/package=meta meta: General Package for Meta-Analysis. R package version 4.9-2. Schwarzer G. http://CRAN.R-project.org/package=meta, 2018.
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings tables'. In: Higgins JP Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S editor(s). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2019
-
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl AE, Skoetz N, Guyatt GH. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane, Draft Version (29 January 2019).
Singhal 1999
-
- Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. New England Journal of Medicine 1999;341(21):1565-71. - PubMed
Sterne 2011
-
- Sterne JA, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Taplitz 2018
-
- Taplitz RA, Kennedy EB, Bow EJ, Crews J, Gleason C, Hawley DK, et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. Journal of Clinical Oncology 2018;36(30):3043-54. - PubMed
The American Cancer Society 2018
-
- The American Cancer Society. Signs and symptoms of multiple myeloma. https://www.cancer.org/cancer/multiple-myeloma/detection-diagnosis-stagi... (accessed 27 August 2018).
Tierney 2007
Waxman 2010
Weisel 2017
-
- Weisel K, Doyen C, Dimopoulos M, Yee A, Lahuerta JJ, Martin A, et al. A systematic literature review and network meta-analysis of treatments for patients with untreated multiple myeloma not eligible for stem cell transplantation. Leukemia & Lymphoma 2017;58(1):153-61. - PubMed
Windebank 2008
-
- Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. Journal of the Peripheral Nervous System 2008;13(1):27-46. - PubMed
World Health Organization 2019
-
- World Health Organization. WHO updates global guidance on medicines and diagnostic tests to address health challenges, prioritize highly effective therapeutics, and improve affordable access.. Available at: https://www.who.int/news-room/detail/09-07-2019-who-updates-global-guida... (accessed: 30 August 2019) 2019.
World Health Organization 2019a
-
- World Health Organization. Executive Summary. The Selection and Use of Essential Medicines 2019. Report of the 22nd WHO Expert Committee on the 2019 Selection and Use of Essential Medicines.. Available at: https://apps.who.int/iris/bitstream/handle/10665/325773/WHO-MVP-EMP-IAU-... (accessed 30 August 2019).
References to other published versions of this review
Piechotta 2018
-
- Piechotta V, Jakob T, Scheckel B, Langer P, Monsef I, Scheid, C, et al. Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first-line treatment in adults with transplant-ineligible multiple myeloma: a network meta-analysis. PROSPERO 2018 CRD42018118092 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018118092. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

