Nivolumab Is Effective in Mismatch Repair-Deficient Noncolorectal Cancers: Results From Arm Z1D-A Subprotocol of the NCI-MATCH (EAY131) Study
- PMID: 31765263
- PMCID: PMC6968795
- DOI: 10.1200/JCO.19.00818
Nivolumab Is Effective in Mismatch Repair-Deficient Noncolorectal Cancers: Results From Arm Z1D-A Subprotocol of the NCI-MATCH (EAY131) Study
Abstract
Purpose: The National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) trial, the largest national precision oncology study to date (> 1,100 sites) of patients with relapsed or refractory malignancies, assigned patients to targeted therapy in parallel phase II studies based on tumor molecular alterations. The anti-programmed death receptor 1 inhibitor nivolumab previously showed activity in mismatch repair (MMR)-deficient colon cancer. We hypothesized that nivolumab would have activity in patients with MMR-deficient, noncolorectal tumors.
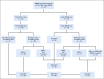
Patients and methods: Eligible patients with relapsed or refractory tumors, good end-organ function, and Eastern Cooperative Oncology Group performance status of ≤ 1 underwent tumor biopsy for centralized screening of molecular alterations. MMR deficiency was defined by complete loss of nuclear expression of MLH1 or MSH2 MMR gene products by immunohistochemistry (IHC). Patients with MMR-deficient colorectal cancer were excluded. Nivolumab, 3 mg/kg every 2 weeks (28-day cycles) and 480 mg every 4 weeks after cycle 4, was administered intravenously. Disease reassessment was performed every 2 cycles. The primary end point was RECIST 1.1 objective response rate (ORR).
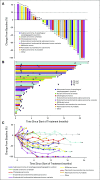
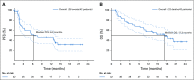
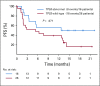
Results: Two percent of 4,902 screened patients had an MMR-deficient cancer by IHC. Forty-two evaluable patients were enrolled, with a median age of 60 years and a median of 3 prior therapies. The most common histologies were endometrioid endometrial adenocarcinoma (n = 13), prostate adenocarcinoma (n = 5), and uterine carcinosarcoma (n = 4). ORR was 36% (15 of 42 patients). An additional 21% of patients had stable disease. The estimated 6-, 12-, and 18-month progression-free survival rates were 51.3% (90% CI, 38.2% to 64.5%), 46.2% (90% CI, 33.1% to 59.3%), and 31.4% (90% CI, 18.7% to 44.2%), respectively. Median overall survival was 17.3 months. Toxicity was predominantly low grade.
Conclusion: A variety of refractory cancers (2.0% of those screened) had MMR deficiency as defined in NCI-MATCH. Nivolumab has promising activity in MMR-deficient noncolorectal cancers of a wide variety of histopathologic types.
Conflict of interest statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Figures

Comment in
-
Has Mismatch Repair-Deficient Cancer Met Its MATCH?J Clin Oncol. 2020 Jan 20;38(3):183-187. doi: 10.1200/JCO.19.02860. Epub 2019 Dec 3. J Clin Oncol. 2020. PMID: 31794325 No abstract available.
References
-
- Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–167. - PubMed
-
- Constantinidou A, Alifieris C, Trafalis DT: Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacol Ther 194:84-106, 2019. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- UG1 CA189821/CA/NCI NIH HHS/United States
- U10 CA180802/CA/NCI NIH HHS/United States
- UG1 CA233341/CA/NCI NIH HHS/United States
- U10 CA180798/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA180844/CA/NCI NIH HHS/United States
- U10 CA180858/CA/NCI NIH HHS/United States
- UL1 TR003167/TR/NCATS NIH HHS/United States
- U10 CA180870/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

