Novel charged sodium and calcium channel inhibitor active against neurogenic inflammation
- PMID: 31765298
- PMCID: PMC6877086
- DOI: 10.7554/eLife.48118
Novel charged sodium and calcium channel inhibitor active against neurogenic inflammation
Abstract
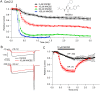
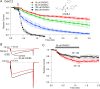
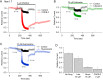
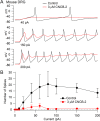
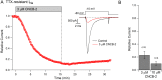
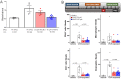
Voltage-dependent sodium and calcium channels in pain-initiating nociceptor neurons are attractive targets for new analgesics. We made a permanently charged cationic derivative of an N-type calcium channel-inhibitor. Unlike cationic derivatives of local anesthetic sodium channel blockers like QX-314, this cationic compound inhibited N-type calcium channels more effectively with extracellular than intracellular application. Surprisingly, the compound is also a highly effective sodium channel inhibitor when applied extracellularly, producing more potent inhibition than lidocaine or bupivacaine. The charged inhibitor produced potent and long-lasting analgesia in mouse models of incisional wound and inflammatory pain, inhibited release of the neuropeptide calcitonin gene-related peptide (CGRP) from dorsal root ganglion neurons, and reduced inflammation in a mouse model of allergic asthma, which has a strong neurogenic component. The results show that some cationic molecules applied extracellularly can powerfully inhibit both sodium channels and calcium channels, thereby blocking both nociceptor excitability and pro-inflammatory peptide release.
Keywords: Cav2.2; Nav1.7; asthma; calcitonin gene-related peptide; dorsal root ganglion; inflammatory peptide; mouse; neuroscience.
© 2019, Lee et al.
Conflict of interest statement
SL, JL, CW, BB is named as an inventor on a patent application (U.S. Patent Office No. 62/769,420) related to this work, SJ, ST, HZ, MK, NA, MP, PL, TJ, MP, LH, AJ No competing interests declared
Figures



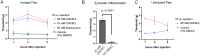
References
-
- Abbadie C, McManus OB, Sun SY, Bugianesi RM, Dai G, Haedo RJ, Herrington JB, Kaczorowski GJ, Smith MM, Swensen AM, Warren VA, Williams B, Arneric SP, Eduljee C, Snutch TP, Tringham EW, Jochnowitz N, Liang A, Euan MacIntyre D, McGowan E, Mistry S, White VV, Hoyt SB, London C, Lyons KA, Bunting PB, Volksdorf S, Duffy JL. Analgesic effects of a substituted N-triazole oxindole (TROX-1), a state-dependent, voltage-gated calcium channel 2 blocker. Journal of Pharmacology and Experimental Therapeutics. 2010;334:545–555. doi: 10.1124/jpet.110.166363. - DOI - PubMed
-
- Ahuja S, Mukund S, Deng L, Khakh K, Chang E, Ho H, Shriver S, Young C, Lin S, Johnson JP, Wu P, Li J, Coons M, Tam C, Brillantes B, Sampang H, Mortara K, Bowman KK, Clark KR, Estevez A, Xie Z, Verschoof H, Grimwood M, Dehnhardt C, Andrez JC, Focken T, Sutherlin DP, Safina BS, Starovasnik MA, Ortwine DF, Franke Y, Cohen CJ, Hackos DH, Koth CM, Payandeh J. Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science. 2015;350:aac5464. doi: 10.1126/science.aac5464. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS072040/NS/NINDS NIH HHS/United States
- R01 NS064274/NS/NINDS NIH HHS/United States
- Blavatnik Biomedical Accelerator Fund/Harvard Medical School/International
- R35 NS105076/NS/NINDS NIH HHS/United States
- R37 NS036855/NS/NINDS NIH HHS/United States
- HR0011-19-2-0022/Defense Advanced Research Projects Agency/International
- P01 NS072040/NS/NINDS NIH HHS/United States
- Boston Children's Hospital's Technology Development Fund/Boston Children's Hospital/International
- R01 NS110860/NS/NINDS NIH HHS/United States
- R01 NS036855/NS/NINDS NIH HHS/United States
- 105076/NS/NINDS NIH HHS/United States
- 110860/NS/NINDS NIH HHS/United States
- NS036855/NS/NINDS NIH HHS/United States
- NS064274/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

