Effect of Carbon Dioxide Loading on Removal of Heat Stable Salts from Amine Solvent by Electrodialysis
- PMID: 31766157
- PMCID: PMC6918454
- DOI: 10.3390/membranes9110152
Effect of Carbon Dioxide Loading on Removal of Heat Stable Salts from Amine Solvent by Electrodialysis
Abstract
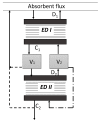
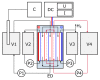
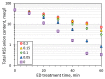
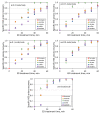
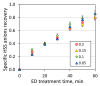
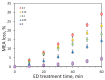
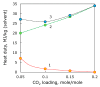
Heat stable salts (HSS) formed and continuously accumulated in the amine-based solvents due to solvent degradation and impurities in the feed gas can dramatically change the efficiency of the amine scrubbing process. HSS can be removed by using different methods including membrane separation such as electrodialysis (ED). In this work, we studied the effect of CO2 loading of the lean 30 wt % monoethanolamine (MEA) solution on the efficiency of HSS removal and MEA loss. In the model MEA solution containing HSS on the level of 48 meq/L, the carbon dioxide concentration was varied from 0.2 down to 0 mole (CO2)/mole (MEA). The reclaiming of model MEA solution was carried out by lab-scale two-stage ED unit when the concentrate stream after the first stage was additionally treated using ED (second stage) that allowed reducing MEA loss. It was shown that the decrease of carbon dioxide content from 0.2 down to 0 mole (CO2)/mole (MEA) resulted in a substantial reduction of both parameters-the MEA loss and the specific power consumption with respect to extracted HSS (from 140 down 37 kJ per 1 g of recovered HSS anions). This can be explained by the drop in the total concentration of ions formed by the interaction of MEA solution with carbon dioxide. However, the change of CO2 loading is associated with additional power consumption towards further solvent regeneration in the column. Based on the preliminary estimations of power consumption required for additional CO2 stripping with the respect to the power consumption of ED stage, it seems that lean solvent CO2 loading of 0.1 mole/mole provides an optimum for the power input at 25.9 MJ/kg(solvent).
Keywords: carbon dioxide; electrodialysis; heat stable salts; monoethanolamine; reclaiming.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Liang Z., Liu H., Fu K., Gao H., Cao F., Zhang R., Sema T., Henni A., Sumon K., Nath D., et al. Recent progress and new developments in post-combustion carbon-capture technology with amine based solvents. Int. J. Greenh. Gas Con. 2015;40:26–54. doi: 10.1016/j.ijggc.2015.06.017. - DOI
-
- Wang M., Joel A.S., Ramshaw C., Eimer D., Musa N.M. Process intensification for post-combustion CO2 capture with chemical absorption: A critical review. Appl. Energy. 2015;158:275–291. doi: 10.1016/j.apenergy.2015.08.083. - DOI
-
- Supap T., Saiwan C., Idem R., Tontiwachwuthikul P. Part 2: Solvent management: Solvent stability and amine degradation in CO2 capture processes. Carbon Manag. 2011;2:551–566. doi: 10.4155/cmt.11.55. - DOI
-
- Verma N., Verma A. Amine system problems arising from heat stable salts and solutions to improve system performance. Fuel Process. Technol. 2009;90:483–489. doi: 10.1016/j.fuproc.2009.02.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

