Surface Morphology-Dependent Functionality of Titanium Dioxide-Nickel Oxide Nanocomposite Semiconductors
- PMID: 31766325
- PMCID: PMC6956268
- DOI: 10.3390/nano9121651
Surface Morphology-Dependent Functionality of Titanium Dioxide-Nickel Oxide Nanocomposite Semiconductors
Abstract
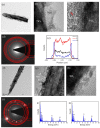
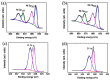
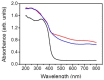
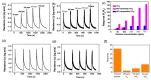
In this study, TiO2-NiO heterostructures were synthesized by combining hydrothermal and chemical bath deposition methods. The post-annealing temperature was varied to control the surface features of the TiO2-NiO heterostructures. TiO2-NiO heterostructures annealed at 350 °C comprised NiO-nanosheet-decorated TiO2 nanostructures (NST), whereas those annealed at 500 °C comprised NiO-nanoparticle-decorated TiO2 nanostructures (NPT). The NPT exhibited higher photodegradation activity than the NST in terms of methylene blue (MB) degradation under irradiation. Structural analyses demonstrated that the NPT had a higher surface adsorption capability for MB dyes and superior light-harvesting ability; thus, they exhibited greater photodegradation ability toward MB dyes. In addition, the NST showed high gas-sensing responses compared with the NPT when exposed to acetone vapor. This result was attributable to the higher number of oxygen-deficient regions on the surfaces of the NST, which increased the amount of surface-chemisorbed oxygen species. This resulted in a relatively large resistance variation for the NST when exposed to acetone vapor.
Keywords: functionality; morphology; semconductors; surface.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Luna-Flores A., Sosa-Sánchez J.L., Morales-Sánchez M.A., Agustín-Serrano R., Luna-López J.A. An Easy-Made, Economical and Efficient Carbon-Doped Amorphous TiO2 Photocatalyst Obtained by Microwave Assisted Synthesis for the Degradation of Rhodamine B. Materials. 2017;10:1447. doi: 10.3390/ma10121447. - DOI - PMC - PubMed
-
- Liang Y.C., Wang C.C. Hydrothermally derived zinc sulfide sphere-decorated titanium dioxide flower-like composites and their enhanced ethanol gas-sensing performance. J. Alloy Compd. 2018;730:333–341. doi: 10.1016/j.jallcom.2017.09.313. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

