Doxorubicin·Hydrochloride/Cisplatin-Loaded Hydrogel/Nanosized (2-Hydroxypropyl)-Beta-Cyclodextrin Local Drug-Delivery System for Osteosarcoma Treatment In Vivo
- PMID: 31766334
- PMCID: PMC6956151
- DOI: 10.3390/nano9121652
Doxorubicin·Hydrochloride/Cisplatin-Loaded Hydrogel/Nanosized (2-Hydroxypropyl)-Beta-Cyclodextrin Local Drug-Delivery System for Osteosarcoma Treatment In Vivo
Abstract
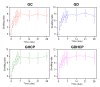
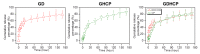
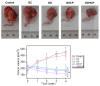
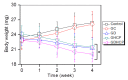
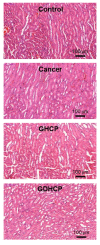
Osteosarcoma (OSA) is a difficult cancer to treat due to its tendency for relapse and metastasis; advanced methods are therefore required for OSA treatment. In this study, we prepared a local drug-delivery system for OSA treatment based on doxorubicin·hydrochloride (DOX·HCl)/cisplatin (CP)-loaded visible light-cured glycol chitosan (GC) hydrogel/(2-hydroxypropyl)-beta-cyclodextrin (GDHCP), and compared its therapeutic efficiency with that of DOX·HCl- and CP-loaded GC hydrogels (GD and GHCP). Because of diffusion driven by concentration gradients in the swollen matrix, the three hydrogels showed sustained releases of DOX·HCl and CP over 7 days, along with initial 3-h bursts. Results of in vitro cell viability and in vivo animal testing revealed that GDHCP had a stronger anticancer effect than GD and GHCP even though there were no significant differences. Body weight measurement and histological evaluations demonstrated that the drug-loaded GC hydrogels had biocompatibility without cardiotoxicity or nephrotoxicity. These results suggested that GDHCP could be a good platform as a local drug-delivery system for clinical use in OSA treatment.
Keywords: (2-hydroxypropyl)-beta-cyclodextrin; cardiotoxicity; cisplatin; doxorubicin·hydrochloride; local drug delivery system; nephrotoxicity; osteosarcoma; visible light-cured glycol chitosan hydrogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ebb D., Meyers P., Grier H., Bernstein M., Gorlick R., Lipshultz S.E., Krailo M., Devidas M., Barkauskas D.A., Siegal G.P., et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: A report from the children’s oncology group. J. Clin. Oncol. 2012;30:2545–2551. doi: 10.1200/JCO.2011.37.4546. - DOI - PMC - PubMed
-
- Au K.M., Satterlee A., Min Y., Tian X., Kim Y.S., Caster J.M., Zhang L., Zhang T., Huang L., Wang A.Z. Folate-targeted pH-responsive calcium zoledronate nanoscale metal-organic frameworks: Turning a bone antiresorptove agent into an anticancer therapeutic. Biomaterials. 2016;82:178–193. doi: 10.1016/j.biomaterials.2015.12.018. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

