mTOR Links Tumor Immunity and Bone Metabolism: What are the Clinical Implications?
- PMID: 31766386
- PMCID: PMC6928935
- DOI: 10.3390/ijms20235841
mTOR Links Tumor Immunity and Bone Metabolism: What are the Clinical Implications?
Abstract
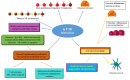
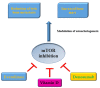
Phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) plays a crucial role in the control of cellular growth, proliferation, survival, metabolism, angiogenesis, transcription, and translation. In most human cancers, alterations to this pathway are common and cause activation of other downstream signaling pathways linked with oncogenesis. The mTOR pathway modulates the interactions between the stroma and the tumor, thereby affecting both tumor immunity and angiogenesis. Inflammation is a hallmark of cancer, playing a central role in the tumor dynamics, and immune cells can exert antitumor functions or promote the growth of cancer cells. In this context, mTOR may regulate the activity of macrophages and T cells by regulating the expression of cytokines/chemokines, such as interleukin (IL)-10 and transforming growth factor (TGF-β), and/or membrane receptors, such as cytotoxic T-Lymphocyte protein 4 (CTLA-4) and Programmed Death 1 (PD-1). Furthermore, inhibitors of mammalian target of rapamycin are demonstrated to actively modulate osteoclastogenesis, exert antiapoptotic and pro-differentiative activities in osteoclasts, and reduce the number of lytic bone metastases, increasing bone mass in tumor-bearing mice. With regard to the many actions in which mTOR is involved, the aim of this review is to describe its role in the immune system and bone metabolism in an attempt to identify the best strategy for therapeutic opportunities in the metastatic phase of solid tumors.
Keywords: bone; everolimus; mTOR; mTOR inhibitors; metastasis; osteoimmunology; tumor immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

