The Efficiency of Color Space Channels to Quantify Color and Color Intensity Change in Liquids, pH Strips, and Lateral Flow Assays with Smartphones
- PMID: 31766483
- PMCID: PMC6928750
- DOI: 10.3390/s19235104
The Efficiency of Color Space Channels to Quantify Color and Color Intensity Change in Liquids, pH Strips, and Lateral Flow Assays with Smartphones
Abstract
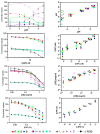
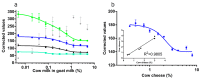
Bottom-up, end-user based feed, and food analysis through smartphone quantification of lateral flow assays (LFA) has the potential to cause a paradigm shift in testing capabilities. However, most developed devices do not test the presence of and implications of inter-phone variation. Much discussion remains regarding optimum color space for smartphone colorimetric analyses and, an in-depth comparison of color space performance is missing. Moreover, a light-shielding box is often used to avoid variations caused by background illumination while the use of such a bulky add-on may be avoidable through image background correction. Here, quantification performance of individual channels of RGB, HSV, and LAB color space and ΔRGB was determined for color and color intensity variation using pH strips, filter paper with dropped nanoparticles, and colored solutions. LAB and HSV color space channels never outperformed the best RGB channels in any test. Background correction avoided measurement variation if no direct sunlight was used and functioned more efficiently outside a light-shielding box (prediction errors < 5%/35% for color/color intensity change). The system was validated using various phones for quantification of major allergens (i.e., gluten in buffer, bovine milk in goat milk and goat cheese), and, pH in soil extracts with commercial pH strips and LFA. Inter-phone variation was significant for LFA quantification but low using pH strips (prediction errors < 10% for all six phones compared). Thus, assays based on color change hold the strongest promise for end-user adapted smartphone diagnostics.
Keywords: allergens; background correction; color space; food contaminant screening; image correction; lateral flow assay quantification; point of site analyses; smartphone colorimetrics.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures










References
-
- The Rapid Alert System for Food and Feed (2017 Annual Report) [(accessed on 20 November 2019)]; Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_repo....
-
- Sivadasan S., Efstathiou J., Calinescu A., Huatuco L.H. Advances on measuring the operational complexity of supplier-customer systems. Eur. J. Oper. Res. 2006;171:208–226. doi: 10.1016/j.ejor.2004.08.032. - DOI
-
- Knowles T., Moody R., McEachern M. European food scares and their impact on EU food policy. Br. Food J. 2007;109:43–67. doi: 10.1108/00070700710718507. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

