3D Bioprinting of Novel Biocompatible Scaffolds for Endothelial Cell Repair
- PMID: 31766610
- PMCID: PMC6960937
- DOI: 10.3390/polym11121924
3D Bioprinting of Novel Biocompatible Scaffolds for Endothelial Cell Repair
Abstract
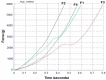
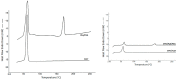
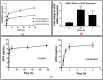
The aim of this study was to develop and evaluate an optimized 3D bioprinting technology in order to fabricate novel scaffolds for the application of endothelial cell repair. Various biocompatible and biodegradable macroporous scaffolds (D = 10 mm) with interconnected pores (D = ~500 µm) were fabricated using a commercially available 3D bioprinter (r3bEL mini, SE3D, USA). The resolution of the printing layers was set at ~100 µm for all scaffolds. Various compositions of polylactic acid (PLA), polyethylene glycol (PEG) and pluronic F127 (F127) formulations were prepared and optimized to develop semi-solid viscous bioinks. Either dimethyloxalylglycine (DMOG) or erythroprotein (EPO) was used as a model drug and loaded in the viscous biocompatible ink formulations with a final concentration of 30% (w/w). The surface analysis of the bioinks via a spectroscopic analysis revealed a homogenous distribution of the forming materials throughout the surface, whereas SEM imaging of the scaffolds showed a smooth surface with homogenous macro-porous texture and precise pore size. The rheological and mechanical analyses showed optimum rheological and mechanical properties of each scaffold. As the drug, DMOG, is a HIF-1 inducer, its release from the scaffolds into PBS solution was measured indirectly using a bioassay for HIF-1α. This showed that the release of DMOG was sustained over 48 h. The release of DMOG was enough to cause a significant increase in HIF-1α levels in the bioassay, and when incubated with rat aortic endothelial cells (RAECs) for 2 h resulted in transcriptional activation of a HIF-1α target gene (VEGF). The optimum time for the increased expression of VEGF gene was approximately 30 min and was a 3-4-fold increase above baseline. This study provides a proof of concept, that a novel bioprinting platform can be exploited to develop biodegradable composite scaffolds for potential clinical applications in endothelial cell repair in cardiovascular disease (CVD), or in other conditions in which endothelial damage occurs.
Keywords: 3D bioprinting; DMOG; EPO; biocompatible; endothelial cell; polylactic acid; scaffolds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
3D-porous β-tricalcium phosphate-alginate-gelatin scaffold with DMOG delivery promotes angiogenesis and bone formation in rat calvarial defects.J Mater Sci Mater Med. 2018 Dec 18;30(1):1. doi: 10.1007/s10856-018-6202-x. J Mater Sci Mater Med. 2018. PMID: 30564959
-
Cell-Laden Nanocellulose/Chitosan-Based Bioinks for 3D Bioprinting and Enhanced Osteogenic Cell Differentiation.ACS Appl Bio Mater. 2021 Mar 15;4(3):2342-2353. doi: 10.1021/acsabm.0c01108. Epub 2021 Feb 17. ACS Appl Bio Mater. 2021. PMID: 35014355
-
Gradient Poly(ethylene glycol) Diacrylate and Cellulose Nanocrystals Tissue Engineering Composite Scaffolds via Extrusion Bioprinting.Front Bioeng Biotechnol. 2019 Oct 18;7:280. doi: 10.3389/fbioe.2019.00280. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31681754 Free PMC article.
-
Biomaterials in bone and mineralized tissue engineering using 3D printing and bioprinting technologies.Biomed Phys Eng Express. 2021 Oct 7;7(6). doi: 10.1088/2057-1976/ac21ab. Biomed Phys Eng Express. 2021. PMID: 34438382 Review.
-
Advances in bioinks and in vivo imaging of biomaterials for CNS applications.Acta Biomater. 2019 Sep 1;95:60-72. doi: 10.1016/j.actbio.2019.05.006. Epub 2019 May 8. Acta Biomater. 2019. PMID: 31075514 Review.
Cited by
-
3D Printed Calcium Phosphate Cement (CPC) Scaffolds for Anti-Cancer Drug Delivery.Pharmaceutics. 2020 Nov 11;12(11):1077. doi: 10.3390/pharmaceutics12111077. Pharmaceutics. 2020. PMID: 33187124 Free PMC article.
-
Reasoning on Pore Terminology in 3D Bioprinting.Gels. 2024 Feb 19;10(2):153. doi: 10.3390/gels10020153. Gels. 2024. PMID: 38391483 Free PMC article. Review.
-
Sustained dual delivery of metronidazole and viable Lactobacillus crispatus from 3D-printed silicone shells.Biomater Adv. 2024 Dec;165:214005. doi: 10.1016/j.bioadv.2024.214005. Epub 2024 Aug 26. Biomater Adv. 2024. PMID: 39208497
-
1Biomaterial inks for extrusion-based 3D bioprinting: Property, classification, modification, and selection.Int J Bioprint. 2022 Dec 9;9(2):649. doi: 10.18063/ijb.v9i2.649. eCollection 2023. Int J Bioprint. 2022. PMID: 37065674 Free PMC article.
-
A Review of Vat Photopolymerization Technology: Materials, Applications, Challenges, and Future Trends of 3D Printing.Polymers (Basel). 2021 Feb 17;13(4):598. doi: 10.3390/polym13040598. Polymers (Basel). 2021. PMID: 33671195 Free PMC article. Review.
References
-
- Maniruzzaman M. 3D and 4D Printing Technology in Biomedical Applications: Process Engineering and Additive Manufacturing. Wiley and Sons; Chichester, West Sussex, UK: 2019.
LinkOut - more resources
Full Text Sources
Research Materials

