S100P is a molecular determinant of E-cadherin function in gastric cancer
- PMID: 31767037
- PMCID: PMC6878717
- DOI: 10.1186/s12964-019-0465-9
S100P is a molecular determinant of E-cadherin function in gastric cancer
Abstract
Background: E-cadherin has been awarded a key role in the aetiology of both sporadic and hereditary forms of gastric cancer. In this study, we aimed to identify molecular interactors that influence the expression and function of E-cadherin associated to cancer.
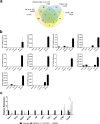
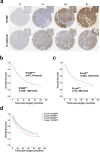
Methods: A data mining approach was used to predict stomach-specific candidate genes, uncovering S100P as a key candidate. The role of S100P was evaluated through in vitro functional assays and its expression was studied in a gastric cancer tissue microarray (TMA).
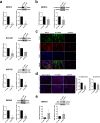
Results: S100P was found to contribute to a cancer pathway dependent on the context of E-cadherin function. In particular, we demonstrated that S100P acts as an E-cadherin positive regulator in a wild-type E-cadherin context, and its inhibition results in decreased E-cadherin expression and function. In contrast, S100P is likely to be a pro-survival factor in gastric cancer cells with loss of functional E-cadherin, contributing to an oncogenic molecular program. Moreover, expression analysis in a gastric cancer TMA revealed that S100P expression impacts negatively among patients bearing Ecad- tumours, despite not being significantly associated with overall survival on its own.
Conclusions: We propose that S100P has a dual role in gastric cancer, acting as an oncogenic factor in the context of E-cadherin loss and as a tumour suppressor in a functional E-cadherin setting. The discovery of antagonist effects of S100P in different E-cadherin contexts will aid in the stratification of gastric cancer patients who may benefit from S100P-targeted therapies.
Keywords: E-cadherin; Gastric cancer; Prognosis; S100P; Survival.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
S100P predicts prognosis and drug resistance in gastric cancer.Int J Biol Markers. 2013 Dec 17;28(4):e387-92. doi: 10.5301/jbm.5000034. Int J Biol Markers. 2013. PMID: 23722300
-
[Impact of S100P expression on clinical outcomes of gastric cancer patients with adjuvant chemotherapy of oxaliplatin and its mechanisms].Zhonghua Wai Ke Za Zhi. 2010 Jul 1;48(13):1004-8. Zhonghua Wai Ke Za Zhi. 2010. PMID: 21054985 Chinese.
-
Knockdown of S100P by lentiviral-mediated RNAi promotes apoptosis and suppresses the colony-formation ability of gastric cancer cells.Oncol Rep. 2014 May;31(5):2344-50. doi: 10.3892/or.2014.3104. Epub 2014 Mar 21. Oncol Rep. 2014. PMID: 24677114
-
E-cadherin and gastric cancer: cause, consequence, and applications.Biomed Res Int. 2014;2014:637308. doi: 10.1155/2014/637308. Epub 2014 Aug 12. Biomed Res Int. 2014. PMID: 25184143 Free PMC article. Review.
-
S100P, a peculiar member of S100 family of calcium-binding proteins implicated in cancer.Acta Virol. 2013;57(2):238-46. doi: 10.4149/av_2013_02_238. Acta Virol. 2013. PMID: 23600880 Review.
Cited by
-
Genetic and Epigenetic Alterations of CDH1 Regulatory Regions in Hereditary and Sporadic Gastric Cancer.Pharmaceuticals (Basel). 2021 May 12;14(5):457. doi: 10.3390/ph14050457. Pharmaceuticals (Basel). 2021. PMID: 34066170 Free PMC article.
-
Calcium-Binding Protein S100P Promotes Tumor Progression but Enhances Chemosensitivity in Breast Cancer.Front Oncol. 2020 Sep 15;10:566302. doi: 10.3389/fonc.2020.566302. eCollection 2020. Front Oncol. 2020. PMID: 33042844 Free PMC article.
-
S100P as a potential biomarker for immunosuppressive microenvironment in pancreatic cancer: a bioinformatics analysis and in vitro study.BMC Cancer. 2023 Oct 18;23(1):997. doi: 10.1186/s12885-023-11490-1. BMC Cancer. 2023. PMID: 37853345 Free PMC article.
-
The Autophagy Machinery Contributes to E-cadherin Turnover in Breast Cancer.Front Cell Dev Biol. 2020 Jun 30;8:545. doi: 10.3389/fcell.2020.00545. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32714931 Free PMC article.
-
The role of PI3K/AKT signaling pathway in gallbladder carcinoma.Am J Transl Res. 2022 Jul 15;14(7):4426-4442. eCollection 2022. Am J Transl Res. 2022. PMID: 35958463 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

