S100A9 regulates porcine reproductive and respiratory syndrome virus replication by interacting with the viral nucleocapsid protein
- PMID: 31767072
- PMCID: PMC7125916
- DOI: 10.1016/j.vetmic.2019.108498
S100A9 regulates porcine reproductive and respiratory syndrome virus replication by interacting with the viral nucleocapsid protein
Abstract
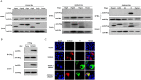
Porcine reproductive and respiratory syndrome virus (PRRSV) has caused huge economic losses to the pig industry worldwide over the last 30 years, yet the associated viral-host interactions remain poorly understood. S100A9 is a damage-associated molecular pattern of the S100 protein family. Here, we found that PRRSV infection stimulated S100A9 expression in porcine alveolar macrophages (PAMs) and Marc-145 cells. S100A9 inhibited PRRSV replication via cellular Ca2+ dependent manner. The viral nucleocapsid (N) protein co-localized with S100A9 in the cytoplasm, and directly interacted at amino acid 78 of S100A9 and amino acids 36-37 of N protein. Moreover, we also found that the mutant S100A9 (E78Q) protein exhibited decreased antiviral activity against PRRSV compared with the parent S100A9. Recombinant PRRSV rBB (36/37) with two mutations in amino acid 36-37 in the N protein exhibited greater replication than the parent PRRSV BB0907 in S100A9-overexpressed PAM and Marc-145 cells. Thus, S100A9 may restrict PRRSV proliferation by interacting with the viral N protein.
Keywords: Inhibit; Nucleocapsid protein; PRRSV; S100A9.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures




References
-
- Chen N., Liu Q., Qiao M., Deng X., Chen X., Sun M. Whole genome characterization of a novel porcine reproductive and respiratory syndrome virus 1 isolate: genetic evidence for recombination between Amervac vaccine and circulating strains in mainland China. Infect. Genet. Evol. 2017;54:308–313. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

