Growth differentiation factor 11 attenuates liver fibrosis via expansion of liver progenitor cells
- PMID: 31767630
- PMCID: PMC7282557
- DOI: 10.1136/gutjnl-2019-318812
Growth differentiation factor 11 attenuates liver fibrosis via expansion of liver progenitor cells
Erratum in
-
Correction: Growth differentiation factor 11 attenuates liver fibrosis via expansion of liver progenitor cells.Gut. 2022 May;71(5):e6. doi: 10.1136/gutjnl-2019-318812corr1. Gut. 2022. PMID: 35396232 Free PMC article. No abstract available.
Abstract
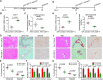
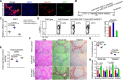
Objective: Liver fibrosis and cirrhosis resulting from chronic liver injury represent a major healthcare burden worldwide. Growth differentiation factor (GDF) 11 has been recently investigated for its role in rejuvenation of ageing organs, but its role in chronic liver diseases has remained unknown. Here, we investigated the expression and function of GDF11 in liver fibrosis, a common feature of most chronic liver diseases.
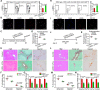
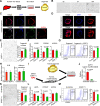
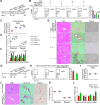
Design: We analysed the expression of GDF11 in patients with liver fibrosis, in a mouse model of liver fibrosis and in hepatic stellate cells (HSCs) as well as in other liver cell types. The functional relevance of GDF11 in toxin-induced and cholestasis-induced mouse models of liver fibrosis was examined by in vivo modulation of Gdf11 expression using adeno-associated virus (AAV) vectors. The effect of GDF11 on leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5)+ liver progenitor cells was studied in mouse and human liver organoid culture. Furthermore, in vivo depletion of LGR5+ cells was induced by injecting AAV vectors expressing diptheria toxin A under the transcriptional control of Lgr5 promoter.
Results: We showed that the expression of GDF11 is upregulated in patients with liver fibrosis and in experimentally induced murine liver fibrosis models. Furthermore, we found that therapeutic application of GDF11 mounts a protective response against fibrosis by increasing the number of LGR5+ progenitor cells in the liver.
Conclusion: Collectively, our findings uncover a protective role of GDF11 during liver fibrosis and suggest a potential application of GDF11 for the treatment of chronic liver disease.
Keywords: GDF11 and LGR5; chronic liver disease; fibrosis; hepatic stellate cells; myofibroblasts; stem cells.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Comment in
-
Harnessing liver progenitors in the treatment of liver fibrosis: a step in the right direction?Gut. 2020 Jun;69(6):975-976. doi: 10.1136/gutjnl-2019-320203. Epub 2020 Jan 20. Gut. 2020. PMID: 31959691 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
