HISTONE DEACETYLASE 9 stimulates auxin-dependent thermomorphogenesis in Arabidopsis thaliana by mediating H2A.Z depletion
- PMID: 31767749
- PMCID: PMC6911240
- DOI: 10.1073/pnas.1911694116
HISTONE DEACETYLASE 9 stimulates auxin-dependent thermomorphogenesis in Arabidopsis thaliana by mediating H2A.Z depletion
Abstract
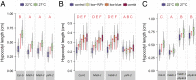
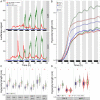
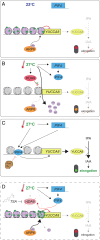
Many plant species respond to unfavorable high ambient temperatures by adjusting their vegetative body plan to facilitate cooling. This process is known as thermomorphogenesis and is induced by the phytohormone auxin. Here, we demonstrate that the chromatin-modifying enzyme HISTONE DEACETYLASE 9 (HDA9) mediates thermomorphogenesis but does not interfere with hypocotyl elongation during shade avoidance. HDA9 is stabilized in response to high temperature and mediates histone deacetylation at the YUCCA8 locus, a rate-limiting enzyme in auxin biosynthesis, at warm temperatures. We show that HDA9 permits net eviction of the H2A.Z histone variant from nucleosomes associated with YUCCA8, allowing binding and transcriptional activation by PHYTOCHROME INTERACTING FACTOR 4, followed by auxin accumulation and thermomorphogenesis.
Keywords: Arabidopsis; H2A.Z; HDA9; shade avoidance; thermomorphogenesis.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Challinor A. J., et al. , A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Chang. 4, 287–291 (2014).
-
- Crawford A. J., McLachlan D. H., Hetherington A. M., Franklin K. A., High temperature exposure increases plant cooling capacity. Curr. Biol. 22, R396–R397 (2012). - PubMed
-
- Quint M., et al. , Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2, 15190 (2016). - PubMed
-
- Casal J. J., Balasubramanian S., Thermomorphogenesis. Annu. Rev. Plant Biol. 70, 321–346 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

