High-throughput assessment of hemoglobin polymer in single red blood cells from sickle cell patients under controlled oxygen tension
- PMID: 31767751
- PMCID: PMC6911208
- DOI: 10.1073/pnas.1914056116
High-throughput assessment of hemoglobin polymer in single red blood cells from sickle cell patients under controlled oxygen tension
Abstract
Sickle cell disease (SCD) is caused by a variant hemoglobin molecule that polymerizes inside red blood cells (RBCs) in reduced oxygen tension. Treatment development has been slow for this typically severe disease, but there is current optimism for curative gene transfer strategies to induce expression of fetal hemoglobin or other nonsickling hemoglobin isoforms. All SCD morbidity and mortality arise directly or indirectly from polymer formation in individual RBCs. Identifying patients at highest risk of complications and treatment candidates with the greatest curative potential therefore requires determining the amount of polymer in individual RBCs under controlled oxygen. Here, we report a semiquantitative measurement of hemoglobin polymer in single RBCs as a function of oxygen. The method takes advantage of the reduced oxygen affinity of hemoglobin polymer to infer polymer content for thousands of RBCs from their overall oxygen saturation. The method enables approaches for SCD treatment development and precision medicine.
Keywords: diagnostics; erythrocytes; hemoglobin polymerization; oxygen affinity; sickle cell disease.
Conflict of interest statement
The authors declare no competing interest.
Figures

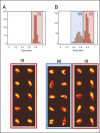

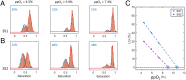
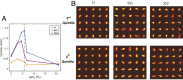
References
-
- Serjeant G. R., Higgs D. R., Hambleton I. R., Elderly survivors with homozygous sickle cell disease. N. Engl. J. Med. 356, 642–643 (2007). - PubMed
-
- Steinberg M. H., Chui D. H. K., Dover G. J., Sebastiani P., Alsultan A., Fetal hemoglobin in sickle cell anemia: A glass half full? Blood 123, 481–485 (2014). - PubMed
-
- Charache S., et al. ; Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia , Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N. Engl. J. Med. 332, 1317–1322 (1995). - PubMed
-
- Platt O. S., et al. , Pain in sickle cell disease. Rates and risk factors. N. Engl. J. Med. 325, 11–16 (1991). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

