A structural basis for antibody-mediated neutralization of Nipah virus reveals a site of vulnerability at the fusion glycoprotein apex
- PMID: 31767754
- PMCID: PMC6911215
- DOI: 10.1073/pnas.1912503116
A structural basis for antibody-mediated neutralization of Nipah virus reveals a site of vulnerability at the fusion glycoprotein apex
Abstract
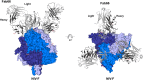
Nipah virus (NiV) is a highly pathogenic paramyxovirus that causes frequent outbreaks of severe neurologic and respiratory disease in humans with high case fatality rates. The 2 glycoproteins displayed on the surface of the virus, NiV-G and NiV-F, mediate host-cell attachment and membrane fusion, respectively, and are targets of the host antibody response. Here, we provide a molecular basis for neutralization of NiV through antibody-mediated targeting of NiV-F. Structural characterization of a neutralizing antibody (nAb) in complex with trimeric prefusion NiV-F reveals an epitope at the membrane-distal domain III (DIII) of the molecule, a region that undergoes substantial refolding during host-cell entry. The epitope of this monoclonal antibody (mAb66) is primarily protein-specific and we observe that glycosylation at the periphery of the interface likely does not inhibit mAb66 binding to NiV-F. Further characterization reveals that a Hendra virus-F-specific nAb (mAb36) and many antibodies in an antihenipavirus-F polyclonal antibody mixture (pAb835) also target this region of the molecule. Integrated with previously reported paramyxovirus F-nAb structures, these data support a model whereby the membrane-distal region of the F protein is targeted by the antibody-mediated immune response across henipaviruses. Notably, our domain-specific sequence analysis reveals no evidence of selective pressure at this region of the molecule, suggestive that functional constraints prevent immune-driven sequence variation. Combined, our data reveal the membrane-distal region of NiV-F as a site of vulnerability on the NiV surface.
Keywords: antibody response; glycoprotein; henipavirus; structure; viral fusion.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Murray K., et al. , A morbillivirus that caused fatal disease in horses and humans. Science 268, 94–97 (1995). - PubMed
-
- Field H. E., Hendra virus ecology and transmission. Curr. Opin. Virol. 16, 120–125 (2016). - PubMed
-
- Paton N. I., et al. , Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet 354, 1253–1256 (1999). - PubMed
-
- Chua K. B., et al. , Nipah virus: A recently emergent deadly paramyxovirus. Science 288, 1432–1435 (2000). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 AI123449/AI/NIAID NIH HHS/United States
- 203141/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MR/L009528/1/MRC_/Medical Research Council/United Kingdom
- R01 GM093939/GM/NIGMS NIH HHS/United States
- MR/N00065X/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- R01 AI125536/AI/NIAID NIH HHS/United States
- F31 AI154739/AI/NIAID NIH HHS/United States
- T32 GM008169/GM/NIGMS NIH HHS/United States
- R01 AI134384/AI/NIAID NIH HHS/United States
- MR/S007555/1/MRC_/Medical Research Council/United Kingdom
- MR/N002091/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

