Molecular Dynamics Simulation reveals the mechanism by which the Influenza Cap-dependent Endonuclease acquires resistance against Baloxavir marboxil
- PMID: 31767949
- PMCID: PMC6877583
- DOI: 10.1038/s41598-019-53945-1
Molecular Dynamics Simulation reveals the mechanism by which the Influenza Cap-dependent Endonuclease acquires resistance against Baloxavir marboxil
Abstract
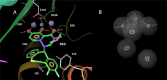
Baloxavir marboxil (BXM), an antiviral drug for influenza virus, inhibits RNA replication by binding to RNA replication cap-dependent endonuclease (CEN) of influenza A and B viruses. Although this drug was only approved by the FDA in October 2018, drug resistant viruses have already been detected from clinical trials owing to an I38 mutation of CEN. To investigate the reduction of drug sensitivity by the I38 mutant variants, we performed a molecular dynamics (MD) simulation on the CEN-BXM complex structure to analyze variations in the mode of interaction. Our simulation results suggest that the side chain methyl group of I38 in CEN engages in a CH-pi interaction with the aromatic ring of BXM. This interaction is abolished in various I38 mutant variants. Moreover, MD simulation on various mutation models and binding free energy prediction by MM/GBSA method suggest that the I38 mutation precludes any interaction with the aromatic ring of BXA and thereby reduces BXA sensitivity.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- WHO. Vaccines against influenza WHO position paper—November 2012. Wkly Epidemiol Rec87, 461–76 (2012). - PubMed
-
- Food and Drug Administration. FDA approves new drug to treat influenza. (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

