Mechanism of ribosome stalling during translation of a poly(A) tail
- PMID: 31768042
- PMCID: PMC6900289
- DOI: 10.1038/s41594-019-0331-x
Mechanism of ribosome stalling during translation of a poly(A) tail
Abstract
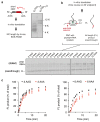
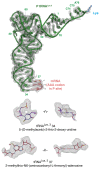
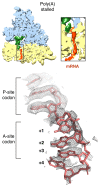
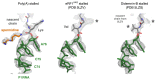
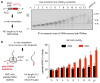
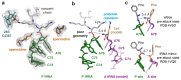
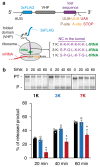
Faulty or damaged messenger RNAs are detected by the cell when translating ribosomes stall during elongation and trigger pathways of mRNA decay, nascent protein degradation and ribosome recycling. The most common mRNA defect in eukaryotes is probably inappropriate polyadenylation at near-cognate sites within the coding region. How ribosomes stall selectively when they encounter poly(A) is unclear. Here, we use biochemical and structural approaches in mammalian systems to show that poly-lysine, encoded by poly(A), favors a peptidyl-transfer RNA conformation suboptimal for peptide bond formation. This conformation partially slows elongation, permitting poly(A) mRNA in the ribosome's decoding center to adopt a ribosomal RNA-stabilized single-stranded helix. The reconfigured decoding center clashes with incoming aminoacyl-tRNA, thereby precluding elongation. Thus, coincidence detection of poly-lysine in the exit tunnel and poly(A) in the decoding center allows ribosomes to detect aberrant mRNAs selectively, stall elongation and trigger downstream quality control pathways essential for cellular homeostasis.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

