Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation
- PMID: 31768073
- PMCID: PMC6923570
- DOI: 10.1038/s41590-019-0539-2
Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation
Abstract
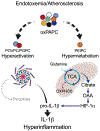
Pathogen-associated molecular patterns (PAMPs) have the capacity to couple inflammatory gene expression to changes in macrophage metabolism, both of which influence subsequent inflammatory activities. Similar to their microbial counterparts, several self-encoded damage-associated molecular patterns (DAMPs) induce inflammatory gene expression. However, whether this symmetry in host responses between PAMPs and DAMPs extends to metabolic shifts is unclear. Here, we report that the self-encoded oxidized phospholipid oxPAPC alters the metabolism of macrophages exposed to lipopolysaccharide. While cells activated by lipopolysaccharide rely exclusively on glycolysis, macrophages exposed to oxPAPC also use mitochondrial respiration, feed the Krebs cycle with glutamine, and favor the accumulation of oxaloacetate in the cytoplasm. This metabolite potentiates interleukin-1β production, resulting in hyperinflammation. Similar metabolic adaptions occur in vivo in hypercholesterolemic mice and human subjects. Drugs that interfere with oxPAPC-driven metabolic changes reduce atherosclerotic plaque formation in mice, thereby underscoring the importance of DAMP-mediated activities in pathophysiological conditions.
Figures







Comment in
-
DAMP-driven metabolic adaptation.Nat Rev Immunol. 2020 Jan;20(1):1. doi: 10.1038/s41577-019-0258-9. Nat Rev Immunol. 2020. PMID: 31792373 No abstract available.
References
-
- Janeway CA Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54 Pt 1, 1–13 (1989). - PubMed
-
- Matzinger P Tolerance, danger, and the extended family. Annu Rev Immunol 12, 991–1045 (1994). - PubMed
-
- Nathan C Points of control in inflammation. Nature 420, 846–852 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

