Tissue-specific tumor microenvironments influence responses to immunotherapies
- PMID: 31768254
- PMCID: PMC6869967
- DOI: 10.1002/cti2.1094
Tissue-specific tumor microenvironments influence responses to immunotherapies
Abstract
Objectives: Investigation of variable response rates to cancer immunotherapies has exposed the immunosuppressive tumor microenvironment (TME) as a limiting factor of therapeutic efficacy. A determinant of TME composition is the tumor location, and clinical data have revealed associations between certain metastatic sites and reduced responses. Preclinical models to study tissue-specific TMEs have eliminated genetic heterogeneity, but have investigated models with limited clinical relevance.
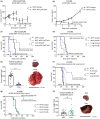
Methods: We investigated the TMEs of tumors at clinically relevant sites of metastasis (liver and lungs) and their impact on αPD-1/αCTLA4 and trimAb (αDR5, α4-1BB, αCD40) therapy responses in the 67NR mouse breast cancer and Renca mouse kidney cancer models.
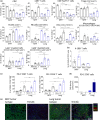
Results: Tumors grown in the lungs were resistant to both therapies whereas the same tumor lines growing in the mammary fat pad (MFP), liver or subcutaneously could be completely eradicated, despite greater tumor burden. Assessment of tumor cells and drug delivery in 67NR lung or MFP tumors revealed no differences and prompted investigation into the immune TME. Lung tumors had a more immunosuppressive TME with increased myeloid-derived suppressor cell infiltration, decreased T cell infiltration and activation, and decreased NK cell activation. Depletion of various immune cell subsets indicated an equivalent role for NK cells and CD8+ T cells in lung tumour control. Thus, targeting T cells with αPD-1/αCTLA4 or trimAb was not sufficient to elicit a robust antitumor response in lung tumors.
Conclusion: Taken together, these data demonstrate that tissue-specific TMEs influence immunotherapy responses and highlight the importance in defining tissue-specific response patterns in patients.
Keywords: anti‐CTLA‐4; anti‐PD‐1; tissue‐specific microenvironment; trimAb; tumor microenvironment.
© 2019 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Pitt JM, Vetizou M, Daillere R et al Resistance mechanisms to immune‐checkpoint blockade in cancer: tumor‐intrinsic and ‐extrinsic factors. Immunity 2016; 44: 1255–1269. - PubMed
-
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21: 309–322. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
